Solutions to 2005 Monthly Questions

Solution to January 2005 Question of the Month
The question was:
How do you stick a clean, bare finger into a beaker of water without getting it wet?
Solution:
Place a magnetic stirring bar into the beaker of water, and then place the beaker on a stirrer.
With the right speed, a vortex will result. You can then place your finger deeply into the
vortex, and it will remain dry.
Solution to February 2005 Question of the Month

The question was:
Over the holidays friends and relatives from Northern Italy served me an almost intolerable amount
of bitter radicchio, which inspired the following question:
Radicchio is a horticultural variety of chicory; both salad greens belong to the same species, Cichorium intybus.
Does that imply that their nutritional value is the same? Hint: examine their colour.
Solution:
Solution to March 2005 Question of the Month
The question was:
This is paraphrased from Calculated Risks by Gerd Gigerenzer, published by Simon and Schuster
in 2002.
One percent of women over 40 have breast cancer, 90% of which test positive on a mammogram.
Of those without breast cancer, however, 9% still test positive. This is the so-called false positive.
Given these statistics, what are the chances that a woman who tests positive actually has breast cancer?
Solution:
Before tackling the problem, consider that nine percent of all women is a large number. And these are women who
test with a false positive on a mammogram. On the other hand, those that both have cancer and test positive are much fewer.
In all likelihood, you will be part of the larger group, so that even if you test positive,
you probably do not have breast cancer. This of course does not apply to all medical tests; it's just that
in the case of the mammogram, the frequency of false positives is too high.
Not convinced?
Consider 1000 women:
| |
|
|
1000 women |
|
|
|
| |
1% of 1000=10 cancer cases |
|
|
|
rest = 990 without cancer |
|
| 1 tests negative |
|
9 cancers test positive |
|
901 normal test negative |
|
89 normal give
false positve |
So out of a total of 89 + 9 = 98 women who test positive, only 9 have cancer. So the likelihood of
both testing positive and having cancer is only 9/98 = about 9%.
Solution to April 2005 Question of the Month
The question was:
How many bonds does the molecule C18H12 contain? Luke Miller obtained the answer without
relying on a Lewis dot structure. Can you?
Solution:
This applies to any neutral organic molecule containing any combination of C,N,O H and halogen atoms.
Luke reasoned that each carbon atom has 4 electrons involved in bonding; N has three; O has 2 and both hydrogen and halogens
have 1 apiece. And because there are two electrons per bond, the total number of bonds, T,is given by
T= (4C + 3N + 2O +1H)/2, where
C = number of carbons in the formula
N = number of nitrogen atoms
O = number of nitrogen atoms
H = total number of hydrogen and halogen atoms
In our example, C18H12, there should be [18(4)+12(1)]/2 = 42 bonds.

Could other information have been extracted from the formula? Certainly.
Before applying the next formula, be sure to do the following:
(1) Drop any oxygens.
(2) Drop one hydrogen along with every nitrogen.
(3) Substitute any halogen with an equal number of hydrogens.
The index of unsaturation = C+ 1 - H/2.
This gives you the total combination of double bonds and ring systems. Triple bonds count as two doubles.
For example, for C18H12, 18 + 1 -(12/2)= 13.
This checks out with the drawn structure, which has 4 rings + 9 double bonds for a total
of 13.
For C5H5N5, after applying rule 2,
we are left with C5,which gives an index of 6, consistent with adenine's structure.
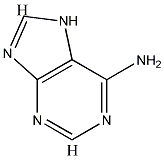
Luke's formula also correctly predicts a total of 40/2 = 20 bonds.
Solution to May 2005 Question of the Month
The question was:
Molecules that were once mainly associated with the atmosphere have turned out to be produced naturally
in our bodies, playing a wide and wild assortment of roles. For instance, in the mid 1980's and early
1990's, NO (nitrous oxide), a common pollutant, was shown to be synthesized in the body from the amino acid (arginine).
Nitrous oxide played the role of a blood vessel dilator, allowing penile erections to materialize.
It also acted as a neurotransmitter in some parts of the brain, permitting peristalsis in the esophagus.
More recently, Scripps Research Institute chemists and others discovered that antibodies appear to
catalyze the formation of ozone(O3). They also showed that this important stratospheric
UV filter but ground-level pollutant is biosynthesized within arteries by atherosclerotic plaques.
In the former case, antibodies first produce the short-lived H2O3 molecule,
which then breaks down in the following overall reaction:
H2O3 --> O2 + H2O2 + O3
Ozone in aqueous systems is short-lived, with a half life of only a minute,
which is probably adequate for killing unwanted invaders.
Notice the reaction is not balanced.
Show that there are several ways of balancing the equation without creating redundant solutions.
Solution:
n H2O3 --> xO2 + n H2O2 + y O3
3n = 2x + 2n + 3y
n = 2x + 3y
if x = y =1, then n = 5;
if x = 2 and y = 1, then n = 7;etc...producing
5 H2O3 --> O2 + 5 H2O2 + O3
or
7 H2O3 --> 2O2 + 7 H2O2 + O3
There are of course many other solutions.Which is the real overall equation? I'm waiting to find out.
Solution to June 2005 Question of the Month
The question was:
Air molecules come in different varieties: nitrogen and oxygen mostly,
with smaller numbers of water, argon, and carbon dioxide molecules. As they constantly bump around,
why can't they sort themselves out? In other words, why don't the lighter H2O gas molecules form an upper
layer, followed by a denser layer of nitrogen, all the way down to carbon dioxide?
Solution:
Solution to July 2005 Question of the Month
The question was:
What came first in the history of medicine: the use of ethyl ether as an anesthetic?
Or the use of phenol (old name was carbolic acid) as an antiseptic?
|
|
ethyl ether(C4H10O) |
phenol(C6H6O) |

Joseph Lister
Solution:
Unfortunately, for patients in the 19th century antiseptics were only used after
the use of ether became common. This general anesthetic had made surgery more popular. The surgeon could
take his time and did not have to rely on strong-armed assistants who were there only to
restrain patients experiencing agonizing pain. But without antiseptics, most people died from infections
following surgery.
Joseph Lister, who had learned about Pasteur's germ theory through a chemistry professor,
had seen cattle recover from an epidemic when carbolic acid(phenol) was added to sewage.
Hospital theatres smelled just like sewage works, so Lister cleaned his theatre with carbolic acid.
He also developed the 'Lister Spray' to spray carbolic acid onto the patient throughout
the operation. This was called anti-septic as the carbolic killed "sepsis" (germs). The spray reduced deaths
during operations by 50%, but was difficult to use. It got into incisions and made
instruments slippery to handle. Surgeons also continued to wear street clothes during operations,
greatly increasing the likelihood of infection.
Further improvements had to await Robert Koch who, in the 1890's, cultured the pus from patients' wounds.
He demonstrated that the pus was caused by bacteria on the surgeons' hands. Through experimentation,
Koch furthermore proved the link between various bacteria and diseases like tuberculosis,
anthrax and cholera, the latter having been one of the major problems associated
with dumping raw sewage into rivers used for
drinking water. This new awareness became a powerful motivator for aseptic surgery:
the whole operating room with its equipment and clothes were germ-free before the operation began.
References:
http://www.bbc.co.uk/schools/gcsebitesize/sosteacher/history/36296.shtml
Burke, James. The Day the Universe Changed. Little Brown & Co. 1986
Solution to August 2005 Question of the Month
The question was:
Why do cooks add salt to water before cooking pasta?
Does the small amount added really
elevate the boiling point of water?
 Solution:
Solution:
Before calculating the answer, it is a lot more fun and more consistent with the ways of science
to actually try it out. Unfortunately our lab is not equipped with pasta; that would have made the
experiment even more rewarding. I took the amount of salt I normally add to a pot of water, weighed it
(6.21g of NaCl) and dissolved it in 425 mL of H2O. I'm sure some people add a little more
salt, but they also start with a lot more water(a couple of litres or so), so in essence, I prepared a more concentrated
solution than what pasta is normally subjected to. To the same amount of the same tap water
I added no salt.
Now one should be suspicious of the thermometers found in most high school labs.
They are scaringly inaccurate. Two thermometers recorded a boiling temperature of about 95 oC; one recorded
a boiling temperature of approximately 106oC. Finally I found a more
reliable mercury one that recorded 100.1 oC
for tap water
and 100.5 oC for the salt solution. So a more concentrated solution of salt
only produced a boiling point elevation(DT) of about 0.4 oC, plus or minus 0.2.
The concentration is directly proportional to boiling point elevation because if water molecules are busy
being attracted to salt ions, it takes even more energy to put them into the former into the gas phase.
According to the theory,
DT= i Kb m,
where i = a constant for a given solute; for NaCl it equals 2.
Kb = constant for the solvent; for water it equals 0.52 °C m-1
m = molality, which is the the number of moles of solute dissolved per kg of solvent
In our case, m = [6.21g/(58.5 g/mole) = 0.1061 moles ]/ 0.425 kg =0.250moles/kg = 0.250 m
DT= (2) (0.52 °C m¯1 ) 0.250 m = 0.26 oC.
Theoretically, the elevation in boiling temperature is within the range of the uncertainty of what I recorded. Moreover,with more
dilute solutions the effect is even less pronounced. If someone typically adds about 10 g of salt in 2.0 L of water, they are looking at a 0.1 oC difference.
For 2 kg of water, that's only an extra 838 J of energy, which can delivered by a 220 V, 8A source
in about half a second. To obtain an increase of a couple of degrees you would need about 200 g
of salt in 2 L of water, not something I would recommend because the pasta will become inedible
! (and unwashable for that matter; an attempt to rinse off the extra salt is futile because it somehow
becomes absorbed and embedded within its structure.)
Solution to September 2005 Question of the Month
The question was:
In chemistry, volumes are not always additive. Can you think of an example for
each of the following three situations?
(1) 19 cm3 of a compound is added to 89 cm3 of an identical compound,
and the level of the liquid only rises to about 96cm3.
(2) An appreciable amount of solid is dissolved in 100.0 ml of water, and the resulting
volume of the solution is less than 100.0 ml.
(3) 10.0 ml of one liquid is added to 5.0 ml of another liquid, the volume of the mixture is less
than 15.0 ml.
Solution:
(1) If ice is added to the water, part of the solid surfaces above the level of the liquid form of water.
Only the submerged part displaces and increases the volume of water.
(2) The solid can be any soluble substance which changes the arrangement of water molecules as they attract and keep ions or
molecules in solution. This arrangement is more orderly and slightly more compact than the free-flowing
form of water.
(3) Alcohol and water also attract each other in solution, creating a mixture with a higher then expected
density. If you could see alcohol molecules, a lot of empty space would appear. Because of H bonding between water and alcohol, water kind
of fills the empty space as it mixes with alcohol. I prepared the following solutions to illustrate the point:
|
Volume of 95% ethanol(ml)
|
V tap H2O added(ml)
|
Volume of mixture removed(ml)
|
total mass(g)
|
mass remaining(g)
|
mass of 10.00 mL sample(g)
|
actual density(g/ml)
|
V mixture(mL)
|
expected density (g/ml)
|
|
10.00
|
0.00
|
10.00
|
8.15
|
|
8.15
|
0.815
|
10.0
|
0.815
|
|
10.00
|
1.00
|
10.00
|
9.14
|
0.64
|
8.50
|
0.850
|
10.8
|
0.831
|
|
10.00
|
2.00
|
10.00
|
10.08
|
1.41
|
8.67
|
0.867
|
11.6
|
0.844
|
|
10.00
|
3.00
|
10.00
|
11.24
|
2.40
|
8.84
|
0.884
|
12.7
|
0.855
|
|
10.00
|
4.00
|
10.00
|
12.15
|
3.22
|
8.93
|
0.893
|
13.6
|
0.865
|
|
10.00
|
5.00
|
10.00
|
13.15
|
4.12
|
9.03
|
0.903
|
14.6
|
0.873
|
|
10.00
|
10.00
|
10.00
|
18.06
|
8.77
|
9.29
|
0.929
|
19.4
|
0.903
|

Solution to October 2005 Question of the Month
The question was:
Is there a connection between the smell of the sea and
the clouds above it?
Solution:
Dead algae release dimethyl sulphide(DMS)which gives the seaside its familiar smell. These
then break down to yield other substances that help seed clouds. More details.
On a slightly related note,compounds known as geosmins are responsible for the earthy smell of organic soil.
They are released by Streptomycete, a type of bacteria and also by some cyanobacteria.
One of these geosmins, trans-1, 10-dimethyl-trans-9-decalol, is actually a type of alcohol, one that our noses are exceptionally
sensitive to, detecting as little as 0.1 parts per billion in the air( 0.1 ppb = 0.1 X 10-6 grams per litre).
References
http://www.thehindu.com/thehindu/seta/2004/09/16/stories/2004091600301500.htm
Budavari,Susan(editor). Merck Index. Twelfth Edition. 1996
Solution to November 2005 Question of the Month
The question was:
 Many books on medicinal plants list chamomile as a beneficial plant.
Is there any pharmacological evidence for this claim?
Many books on medicinal plants list chamomile as a beneficial plant.
Is there any pharmacological evidence for this claim?
Solution:
Recent evidence suggests that compounds in chamomile do have some
antibacterial activity. Details.
Solution to December 2005 Question of the Month
The largest family of flowering plants in the world is the Compositae(Asteracea, or plainly the sunflower family).
In spite of having over 24 000 species, the family does not feature a great deal of edible plants. But a traditional
Chinese medicinal herb, Artemisia annua(sweet wormwood)contains chemicals
that fight malaria more effectively than all other substances. Its compounds also have the unique property of blocking human to mosquito transmission of the parasite
responsible for the deadly disease.
Artemisia annua(sweet wormwood)contains chemicals
that fight malaria more effectively than all other substances. Its compounds also have the unique property of blocking human to mosquito transmission of the parasite
responsible for the deadly disease.
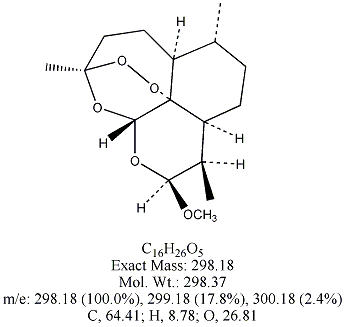
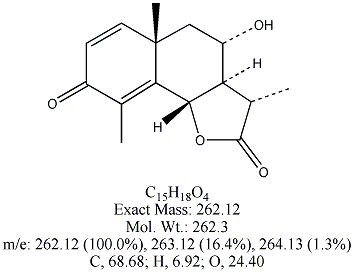
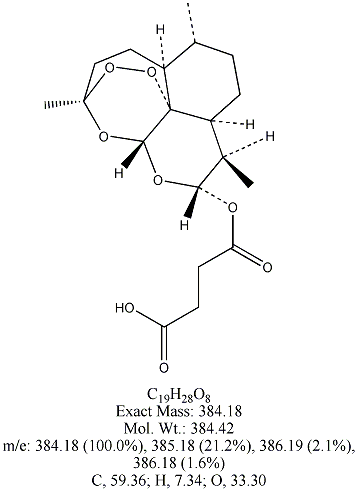
Each of the following four substances is either extracted from the herb or is
a derivative of such a molecule. From similarities in structure, can you guess which three have antimalarial properties?
EXTRA
In school we often absorb information out of historical context.
In reality economic and social conditions often influence scientific research.
Can you match the following historical events with the scientific discoveries
of the times?
| A. Lavoisier published Traitement elementaire de chimie in which he
explained combustion as oxidation and devised modern chemical nomenclature._?_ |
1. Steam locomotive was invented. Britain declared war on France.
U.S. president Jefferson purchased Louisiana from Napoleon, doubling size of U.S.
|
| B. Mendeleev organized elements into a periodic table._?_ |
2. French Revolution began as Bastille was stormed by Parisians
who wanted to free political prisoners. Five yeas later, this scientist
was tragically guillotined
for being indirectly involved in tax-collecting |
| C. Rutherford changed the model of atom by proposing small dense nucleus._?_ |
3. Dostoevsky's writes Crime and Punishment.Alexander II emancipates serfs
and introduces reforms |
| D. Dalton's Atomic Theory was published, revealing that compounds consist of
elements in whole number ratios. _?_ |
4. Beginning of Cubism (Braque; Picasso);Industrial unrest in Britain |
Solution:
All except artemisin. Note that the three antimalarial drugs have a seven-membered ring
embedding a peroxide group(oxygen bonded to oxygen). The latter feature is
what makes the drug deadly to the Plasmodium parasites responsible for invading red blood cells.
References
Budavari,Susan(editor). Merck Index. Twelfth Edition. 1996
Dunavan, Claire Panosian. Malaria: What Must be Done to Stop It. Scientific American. December 2005
EXTRA
A2,B3,C4,D1.
If you like this sort of thing check out this link.
Page Maintained by E. Uva;
euva@retired.ca
Copyright ©2005













 Many books on medicinal plants list chamomile as a beneficial plant.
Is there any pharmacological evidence for this claim?
Many books on medicinal plants list chamomile as a beneficial plant.
Is there any pharmacological evidence for this claim? Artemisia annua(sweet wormwood)contains chemicals
that fight malaria more effectively than all other substances. Its compounds also have the unique property of blocking human to mosquito transmission of the parasite
responsible for the deadly disease.
Artemisia annua(sweet wormwood)contains chemicals
that fight malaria more effectively than all other substances. Its compounds also have the unique property of blocking human to mosquito transmission of the parasite
responsible for the deadly disease.