A piece of aluminum foil is placed in a solution of copper (II) chloride, CuCl2.
Almost instantly a reaction begins as the Cu+2 oxidizes the aluminum.
Someone who has run out of CuCl2 attempts the same reaction using copper sulfate, but it fails.
Why is there no reaction even though copper sulfate also contains Cu+2?
Solution:
Aluminum foil and most aluminum products are normally not reactive because they get covered by a thin oxide layer that fits
nicely on top of the aluminum crystal. As a result oxygen or other oxidizing agents such as Cu+2 cannot steal any more electrons from the aluminum. This is why the copper sulfate fails to react.
But copper chloride contains chloride which penetrates the oxide layer and allows the aluminum underneath to react with
the copper ion.
Solution to October 2007 Question of the Month
The question was:
In the shell model of the nucleus, there are certain magic numbers of nucleons (protons or neutrons): 2, 8, 20, 28, 50, 82, 126 that are more tightly bound than the next higher integer. A magic number of nucleons is important because it increases the likelihood that isotopes will be stable. If the number of neutrons and protons are both magic numbers (not necessarily the same one) we say the nucleus is doubly magic, and it is even more likely to be stable. Isotopes with doubly magic numbers include :
4He |
16O |
40Ca, 48Ca |
56Ni, 48Ni |
132Sn |
208Pb |
None of the above isotopes are radioactive except for 132Sn,
which, in spite of its double magic number of 50 protons and 82 neutrons,
has a half life of less than a day. So a few years ago I contacted a scientist to ask him why 132Sn
is radioactive.
Solution:
Here is the answer from Alex Brown of the National Superconducting Cyclotron Laboratory http://www.nscl.msu.edu
Sn-132 is doubly-magic, but being doubly-magic
does not guarantee stability. There are other features that can
make a nucleus unstable. In this case Sn-132 is too far
from the "valley of stability" - it has too many
neutrons. The neutrons in Sn-132 beta decay
and turn into protons eventally leading to the most stable
mass 132 nucleus Xe-132.
The "magic number" of 50 protons
for Sn does show up by the fact that Sn has more
stable isotopes than any other element - they are
Sn-112, Sn-114, Sn-115, Sn-116, Sn-117, Sn-118,
Sn-119, Sn-120, Sn-122 and Sn-124. There are other nuclei
that we predict to be doubly-magic that are unstable,
such as Sn-100 and Ni-78.
At our National Superconducting Cyclotron Laboratory
are are trying to produce these nuclei and study their properties.
Solution to November 2007 Question of the Month
The question was:
A small amount of acetaminophen, Tylenol's active ingredient,
is toxic to cats but not to dogs or humans. Why?
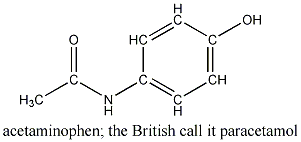
Solution:
After they have served their purpose, drugs in the body are sometimes broken down into other
molecules, and then they are excreted. In acetaminophen's case, animals excrete a combination of
unmetabolized acetaminophen and break down products.
In certain cells, acetaminophen is metabolized to its
highly reactive metabolite N-acetyl-p-benzoquinoneimine(NAPQI). 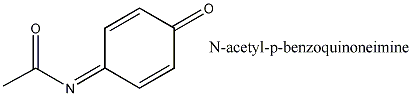
This molecule can kill cells but it is usually picked up by other molecules and gotten rid of before it does too much damage, provided that there isn't too much NAPQI produced.
When the body gets rid of the acetaminophen, even before the reactive metabolites are made, it does so by
using the enzyme glucuronyl transferase which conjugates acetaminophen to glucuronic acid for excretion.
The problem with cats is that they have very little glucuronyl transferase. So instead of leaving their body,
acetaminophen sticks around, gets converted into an excessive and lethal amount of reactive NAPQI.
The lethal dose for a cat is 50 to 100 mg/kg. In humans it is 360 mg/kg. So even an infant of the
weight of a cat can tolerate approximately 3.6 to 7 times more tylenol.
References: http://www.addl.purdue.edu/newsletters/1998/spring/acet.shtml
doi.wiley.com/10.1002/jps.2600630906
http://www.ansci.cornell.edu/plants/toxcat/toxcat.html
Solution to December 2007 Question of the Month
The question was:
What molecule breaks down into acid and oxygen when it loses electrons, but turns into base and hydrogen when it accepts electrons? How is this relevant to basic life processes?
Solution:
Click on electrolysis.
Page Maintained by E.Uva
Comments:
euva@retired.ca or euva@prof.emsb.qc.ca
Copyright ©2007