|
|
The question was: In a McGuyver episode, McGuyver and a friend were trapped in a room that was slowly being filled with hydrogen sulfide gas. To buy some time, our sleuth advised the elderly gentleman to step on a few boxes. Was this sound advice? Why or why not ? Solution:
The molar mass of H2S is 34 g/mole. Air consists of 78% nitrogen(N2) and 21% oxygen (O2), so on the average, its molar mass is about 0.78(28)+ 0.21(32) = 29 g/mole. Because of Avogadro's Hypothesis ( under the same conditions, equal volumes have the same number of particles) a higher molar mass for hydrogen sulfide implies a higher density than that of an oxygen-nitrogen mixture. As a result, before the poison diffuses into the air, the former will stay at ground level, at least for a little while and will buy McGuyver some time . So his advice is sound.
The question was: If it's true that hot pipes freeze faster then cold ones, what could possibly explain why ? Solution:
Hot water contains less air than cold water because the solubility of gases in liquids decreases with increasing temperature. So, ice formed from hot water is more dense than ice formed from cold water if the air does not get a chance to escape as the water cools in a confined area such as a pipe.
( The air is responsible for the white colour that is seen in ice when it forms from cold water. ) This explains why hot water pipes burst faster. The ice may form later; after all hot water does not freeze faster, but the higher density of such ice causes more problems.
The question was: Near the equator, what will a half moon look like at sunset ( Will the diameter separating the illuminated part from the dark-half run perpendicular, or will it run parallel to the horizon ?),and why ? Solution:
It will be parallel to the horizon. When the moon is in its first quarter ( or "half moon" phase), the earth, sun and moon form a 90 degree angle, with the earth at the vertex. At sunset, the moon will be more or less overhead at the equator, depending on the season. Now if you were in the far north, you would be standing more or less parallel to the line separating the dark and illuminated parts of the moon. So it would appear perpendicular to the horizon. But near the equator, your body has now "turned" 90 degrees, so it will appear parallel to the horizon.
The question was: What role does scent play in sex ? Solution:
The classic answer to a tough biological question is " Well, it depends on the organism."
For many insects, one or two compounds are responsible for attracting males to females. These were isolated by using column chromotography, which causes different molecules to flow out of a column at a different rate. When a mixture from tons of mashed up females are passed through a column, eventually the effective compound drips out, and living males go beserk, or at least move closer to the source. Later somebody got the clever idea of applying these attractants or pheromones to a gluey surface, therefore trapping undesirable insects. So we know such sex attractants exist in insects.
Humans tend to be a little more complicated. Men with blocked noses ( I, for one) can be strongly attracted to their wives, and either sex can be stimulated by just a painting, image or sculpture--let alone actual personality traits, suggesting our selection is based on more than just smell.
The question was:
What would a burning candle look like in the absence of gravity ? Solution:
This experiment was carried out on the space station Mir ( see Scientific American May 1998, p49). After ignition , the candle flame was hemispherical with a bright yellow core.Because there was no convection ( no gravity meant that there was no difference in density between warm and cooler air) less oxygen reached the flame, so the wax burnt five times more slowly than on earth. At the same time because heat wasn't rising, the entire wax melted within a couple of minutes.
The question was:
A detective walked into the prime minister's home where poor Mr Chretien had been tied up while his home had been burglarized. "I don't believe you'll find too many clues", said Chretien. "The man was masked, gloved and meticulous enough to vacuum and scrub the place as to not leave any hair or skin cells for modern forensic analysis. I even heard him brush the toilet bowl for five minutes after urinating. And then he flushed two or three more times after that." The last detail caught the detective's attention. There is usually no DNA present in urine, but it was possible that the burglar was unaware of the fact. Nevertheless, he looked around the toilet bowl. Few men realize how much splashing urinating actually causes. The side of Mr. Chretien's green vanity was stained with a few black spots. Laboratory analysis revealed that the spots were not mold but contained urea and black products derived from homogentisic acid. " Well, we definitely know something about the crook", said the forensic expert at the lab. What had they stumbled upon?
Solution:
The burglar suffered from a metabolic disorder known as alcaptonuria. In people without the condition, an enzyme breaks down a derivative of the amino acid phenylalanine(homogentisate) into a product that is eventually broken down into colorless ions. Historically, alcaptonuria is quite significant. Archibald Garrod realized that the condition was not due to bacteria but to some metabolic disorder. Known as black diaper syndrome, it was observed in newborns who Garrod realized lacked bacterial colonies. He also noticed that alcaptonuria is rare in the general population but more common among children of first cousins. In 1995 Spanish scientists found a gene in fungus that codes for homogentisate dioxygenase. In patients with alcaptonuria a single gene has one of a few possible mutations that lead to the production of a dysfunctional enzyme.
The question was: How do babies eyes go from blue to brown ? Solution:
As Britannica puts it," the scattering of light to produce blue colours of structural origin, such as that of eyes or of many feathers, occurs when small air vesicles or
suspended particles scatter the shorter wavelengths of blue light while allowing
the remaining colours to be absorbed by an underlying layer of dark
pigments."
The question was: Is there any basis behind " Red at night, sailors' delight. Solution:
Assuming that many air masses move from west to east, a clear band above the horizon indicates that the incoming air mass in the late evening is dry, and there is a good chance that the next day will be fair. The red clouds above the clear horizon will drift eastward. But if there is a clear band above the eastern horizon at sunrise, that dry air mass will drift away and the clouds that were colored will continue to cover the sky and lead to an overcast day.
The question was:
Solution to February 1998 Puzzle of the
Month
Solution to March 1998 Puzzle of the
Month
Solution to April 1998 Puzzle of the
Month
In carnivores, anal glands are common and play a role in sexual attraction. Male rodents --specifically mice-- undoubtedly detect the presence of a female by smell alone. Just try putting a cardboard between two cages, both containing mice of separate sexes, and watch the behaviour of the male.
In female rhesus monkeys vaginal secretions increase near the halfway point of the estrus cycle and turn on male monkeys.
But to return to the question, Mclintock found that female menstrual cycles can be synchronized when women cohabit, as in dormitories. The chemical responsible came from a female's armpits, which affected another female's cycle in the absence of all other contact. So it seems that although more complex cues have indeed evolved in humans, the primitive sense of smell still has leaves its trace.
Solution to May 1998 Puzzle of the
Month
Solution to June 1998 Puzzle of the
Month 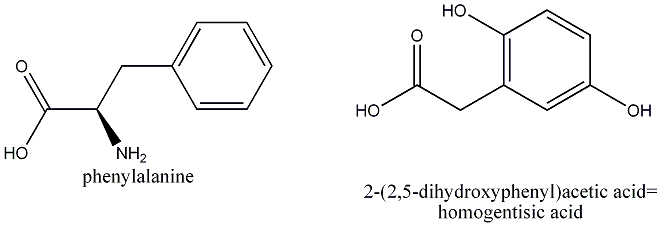 But in people who suffer from the condition, homogentisate accumulates and is excreted in the urine. On standing homogentisate is oxidized and turns into a dark melanin-like substance.
But in people who suffer from the condition, homogentisate accumulates and is excreted in the urine. On standing homogentisate is oxidized and turns into a dark melanin-like substance.
Solution to July 1998 Puzzle of the
Month
If there is very little pigment(melanin) underneath, then irises appear blue. Many babies are born with very little pigment. But then as melanin accumulates, the absorption of other colours masks what ever blue light is scattered. If there is enough melanin present then eyes turn brown; otherwise, if less is deposited, they turn green.
Solution to August 1998 Puzzle of the
Month
Red in the morning, sailors take warning ?
Solution to September 1998 Puzzle of the
Month
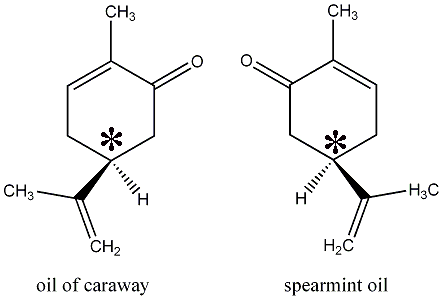
The asterisk represents a carbon atom. The hydrogen attached to this carbon has to be imagined pushing into the paper. The other three carbon atoms that are attached to the * are being projected out. Why then are the two molecules not identical ?
Solution:
The two molecules are said to be "chiral". Whenever four different groups are attached to a carbon atom , and if there is no internal plane of symmetry in the molecule, then the molecule will have a mirror image that is not superimposable. Keep in mind that such a carbon is tetrahedral; only two groups from the mirror image can be matched with the first molecule. Because the arrangement of the atoms in 3-d space is different, they will interact differently with receptors and other molecules. In short, their chemical properties will differ.
Other examples of chiral molecules in nature include the left and right handed versions of limonene. One smells like oranges; the other like lemons. In the early sixties, some pregnant women who took the sedative thalidomide in their first trimester gave birth to deformed babies. The product was sold as a racemic mixture, meaning that chemists did not separate the mixture of chiral molecules. It is unfortunate because what they did not know was that only the right-handed version led to birth defects. The left-handed version of thalidomide would have been a safe sedative.
Solution to October 1998 Puzzle of the
Month
The question was: What evidence exists for the idea that the nebula from which our solar system formed was compressed by the shock wave of a nearby supernova? Solution:
In the 1960's unusual isotope ratios were found in meteorites. For example, carbonaceous meteorites are enriched with oxygen-16. The most reasonable source for the extra O-16 is a supernova that exploded shortly before the formation of the solar system so that the debris containing the particular isotope did not have a chance to mix with the rest of the solar nebula. Meteorites also contain an excess of Mg-26 in aluminum-27 rich rocks. Mg-26 comes from the beta decay of a short lived isotope of aluminum, Al-26( half life 700 000 y), produced in supernovae. If a supernova had exploded long before the condensation of the solar nebula, the Al-26 would have decayed into Mg before chemical segregation, meaning that today we would have found the Mg-26 only in the presence of magnesium rich grains. But instead the Al-26 was injected afterwards, so that it coupled with other Al( isotopes have the same chemical behaviour), and when it did decay, its byproduct, Mg-26 was trapped with aluminum-27.
The question was: Is there really a connection between Vitamin C and cold-prevention, or is it just a myth?
Solution:
No one has been able to substantiate this claim with any clinical evidence. Linus Pauling advocated consumption of large doses of this vitamin, and helped create the myth. Although it is true that many North Americans consume too few fruits and vegetables and therefore don't get enough of this anti-oxidant, too much can potentially lead to problems. For instance one gram, however, is considered a toxic dosage (Drugs, Vitamins and Minerals. Henry and al. Fisher,1989).
The question was:
What makes armpits smell the way they do? Solution:
Solution to November 1998 Puzzle of the
Month
Solution to December 1998 Puzzle of the
Month
It was once believed that butyric acid, the same compound found in rancid butter, was responsible for the foul smell. If that was the case, then one could potentially use alcohol and the proper catalyst to convert butyric acid into ethyl butyrate, an ester that smells like pineapples. But it turns out that the main molecule responsible for body odour is another carboxylic acid, namely 3-methyl 2-hexanoic acid. (Preti, 1990)
Copyright ©2008
|
|
|
:
|