|
Year |
Winner |
Country* |
Achievement |
|
1901 |
Netherlands |
for work on rates of reaction, chemical equilibrium, and osmotic pressure |
||
1902 |
|
Germany |
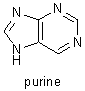
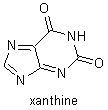
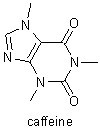
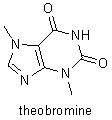
Fischer's research on the purines was instituted in 1881. He determined the structures of uric acid, xanthine, caffeine, theobromine, and other related compounds, and he showed that they are all derivatives of a single compound, a nitrogenous base that he named purine. Despite major complications because of stereochemical relations, Fischer was able to use these derivatives to determine the molecular structures of fructose, glucose, and many other sugars, and he was able to verify his results by synthesizing those compounds. He also showed how to distinguish the formulas of the 16 stereoisometric glucoses. In the course of his stereochemical research, Fischer discovered that there are two series of sugars, the D sugars and the L sugars, that are mirror images of each other. His study of sugars led him to investigate the reactions and substances involved in fermentation, and, in his investigations of how enzymes break down sugars, Fischer laid the foundations for enzyme chemistry. |
|
1903 |
|
Sweden |
theory of
electrolytic dissociation. |
|
1904 |
|
U.K. |
discovery of inert
gas elements and their places in the periodic system. |
|
1905 |
|
Germany |
Notable among Baeyer's many achievements were the discovery of the phthalein dyes(including indigo) and his investigations of uric acid derivatives, polyacetylenes, and oxonium salts. One derivative of uric acid that he discovered was barbituric acid, the parent compound of the sedative-hypnotic drugs known as barbiturates. Baeyer proposed a "strain" (Spannung) theory that helped explain why carbon rings of five or six atoms are so much more common than carbon rings with other numbers of atoms. |
|
1906 |
|
France |
isolation of
fluorine; introduction of Moissan furnace |
|
1907 |
|
Germany |
for demonstrating that the fermentation of carbohydrates results from the action of different enzymes contained in yeast and not the yeast cell itself. He showed that an enzyme, zymase, can be extracted from yeast cells and that it causes sugar to break up into carbon dioxide and alcohol. |
|
1908 |
|
U.K. |
investigations into the disintegration of
elements and the chemistry of radioactive substances |
|
1909 |
|
Germany |
pioneer work on catalysis, chemical
equilibrium, and reaction velocities |
|
1910 |
|
Germany |
|
|
1911 |
|
France |
discovery of radium and polonium; isolation of radium |
|
1912 |
|
France |
discovery of the Grignard reagents, consisting of organometallic compounds in which a negatively polarized carbon can add itself and its adjacent atoms to an organic compound containing an electrophilic site |
|
|
|
France |
for his method of hydrogenating organic compounds in the presence of finely disintegrated metals. In 1897 he reduced ethylene to ethane with hydrogen using nickel as a catalyst and eventually extended his approach to wide variety of organic compounds. |
||
1913 |
|
Switzerland |
for coordination theory, which permitted a simple classification of inorganic compounds and extended the concept of isomerism |
|
1914 |
|
U.S. |
accurate determination of the atomic weights of
numerous elements |
|
1915 |
|
Germany |
pioneer researches in plant pigments, especially chlorophyll |
|
1916-17 |
No Prizes awarded |
|||
1918 |
|
Germany |
In the first decade of the 20th century the rapidly increasing demand for nitrogen fertilizer greatly exceeded the supply, which still came mainly from Chilean nitrate. The problem of utilizing atmospheric nitrogen for this purpose had become of worldwide concern. (See nitrogen fixation.) Haber developed a method for synthesizing ammonia from nitrogen and hydrogen, and by 1909 he had established conditions for the large-scale synthesis of ammonia. The process was handed over to Carl Bosch of Badische Anilin- & Soda-Fabrik for industrial development, leading to the Haber-Bosch process. |
|
1919 |
No Prize awarded |
|||
1920 |
|
Germany |
work in thermochemistry |
|
1921 |
|
U.K. |
chemistry of radioactive substances; occurrence and nature of isotopes |
|
1922 |
|
U.K. |
work with mass spectrograph; whole-number rule |
|
1923 |
|
Austria |
method of microanalysis of organic substances |
|
| 1924 | No Prize awarded | |||
1925 |
|
Austria |
elucidation of the heterogeneous nature of colloidal solutions |
|
1926 |
|
Sweden |
for his studies in the chemistry of colloids and for his invention of the ultracentrifuge, which he used to determine precisely the molecular weights of highly complex proteins such as hemoglobin |
|
1927 |
|
Germany |
for his determination of the molecular structure of bile acids. |
|
1928 |
|
Germany |
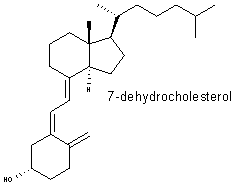
Windaus discovered 7-dehydrocholesterol , which is the chemical precursor of vitamin D, and he showed that it is a steroid. He discovered that it is converted into the vitamin when one of its chemical bonds is broken by the action of sunlight. This explained why exposure to sunlight can prevent vitamin D deficiency (rickets) in humans. Windaus' research also helped establish the chemistry of the sex hormones and advanced the development of drugs used to treat heart ailments |
|
1929 |
|
Sweden |
|
|
|
U.K. |
His more than 20 years of study of the fermentation of sugar advanced knowledge of intermediary metabolic processes in all living forms. He also pioneered in studies of bacterial enzymes and metabolism. | ||
1930 |
|
Germany |
Hemin is a crystalline product of hemoglobin. By splitting in half the molecule of bilirubin, a bile pigment related to hemin, Fischer obtained a new acid in which a section of the hemin molecule was still intact. Fischer identified its structure and found it to be related to pyrrole. This made possible the artificial synthesis of hemin from simpler organic compounds whose structure was known. Fischer also showed that there is a close relationship between hemin and chlorophyll, and by the time of his death he had nearly completed the synthesis of chlorophyll. |
|
1931 |
|
Germany |
invention and development of chemical high-pressure methods. These studies led to his work on converting coal into liquid hydrocarbons. |
|
|
Germany |
|
||
1932 |
|
U.S. |
discoveries and investigations in surface chemistry. Langmuir's studies of molecular films on solid and liquid surfaces opened new fields in colloid research and biochemistry. |
|
1934 |
|
U.S. |
discovery of hydrogen's heavy isotope deuterium |
|
1935 |
|
France |
synthesis of new radioactive elements |
|
| Irène Curie
|
||||
1936 |
|
Netherlands |
for their work on dipole moments and diffraction of X
rays and electrons in gases |
|
1937 |
|
U.K. |
In 1934, with the British chemist Sir Edmund Hirst, he succeeded in synthesizing vitamin C, the first to be artificially produced. This accomplishment not only constituted a valuable addition to knowledge of organic chemistry but also made possible the cheap production of vitamin C (or ascorbic acid, as Haworth called it) for medical purposes. |
|
|
Switzerland |
Karrer's best-known researches were on plant pigments, particularly the yellow ones (carotenoids), which are related to the pigment in carrots. He not only elucidated the chemical structure of the carotenoids but also showed that some of these substances are transformed into vitamin A in the animal body. In 1930 he established the correct formula for carotene-- the chief precursor of vitamin A--and this was the first time that the chemical structure of a vitamin had been established. Shortly afterward he was able to determine the constitution of vitamin A itself. |
||
1938 |
Kuhn, Richard (declined) |
Germany |
Kuhn investigated the structure of compounds related to the carotenoids, the fat-soluble yellow colouring agents widely distributed in nature. He discovered at least eight carotenoids, prepared them in pure form, and determined their constitution. He discovered that one was necessary for the fertilization of certain algae. Simultaneously with Paul Karrer he announced the constitution of vitamin B2 and was the first to isolate a gram of it. With coworkers he also isolated vitamin B6. |
|
1939 |
Butenandt, Adolf (declined) |
Germany |
In 1929, almost simultaneously with Edward A. Doisy in the United States, Butenandt isolated estrone, one of the hormones responsible for sexual development and function in females. |
|
|
Switzerland |
|
||
1943 |
|
Hungary |
for the use of isotopes as tracers in chemical research |
|
1944 |
|
Germany |
for the discovery of the fission of heavy nuclei |
|
1945 |
|
Finland |
Knowing that the fermentation product, lactic acid, increases the acidity of the silage to a point at which destructive fermentation ceases, he developed a procedure (known by his initials, AIV) for adding dilute hydrochloric or sulfuric acid to newly stored silage, thereby increasing the acidity of the fodder beyond that point. In a series of experiments (1928-29), he showed that acid treatment has no adverse effect on the nutritive value and edibility of the fodder and of products derived from animals fed the fodder. |
|
1946 |
|
U.S. |
preparation of enzymes and virus proteins in pure form. He crystallized pepsin, a digestive enzyme present in gastric juice, in 1930 and found that it is a protein, thus resolving the dispute over what enzymes are. Using the same chemical methods, he isolated in 1938 the first bacterial virus (bacteriophage), which he proved to be a nucleoprotein. Northrop also helped isolate and prepare in crystalline form pepsin's inactive precursor pepsinogen (which is converted to the active enzyme through a reaction with hydrochloric acid in the stomach); the pancreatic digestive enzymes trypsin and chymotrypsin; and their inactive precursors trypsinogen and chymotrypsinogen. |
|
|
U.S. |
for the preparation of enzymes and virus proteins in pure form. In 1935 Stanley crystallized tobacco mosaic virus (TMV, the causative agent of a plant disease) and showed that it is a rod-shaped aggregate of protein and nucleic acid molecules. His work enabled other scientists, utilizing methods of X-ray diffraction, to ascertain unambiguously the precise molecular structures and the modes of propagation of several viruses. |
||
|
U.S. |
After crystallizing the enzyme urease in 1926, Sumner went to Stockholm to study with Hans von Euler-Chelpin and Theodor (The) Svedberg. He crystallized the enzyme catalase in 1937 and also contributed to the purification of several other enzymes. |
||
1947 |
|
U.K. |
His most important studies were undertaken on alkaloids; these are complex, naturally occurring, nitrogen-containing compounds that can have profound biochemical effects on living things. Robinson's efforts to determine the chemical reactions that form alkaloids in plants led him to discover the structures of morphine (1925) and strychnine (1946). |
|
1948 |
|
Sweden |
for their researches in electrophoresis and adsorption analysis. He used electrophoretic methods to separate the chemically similar proteins of blood serum |
|
1949 |
|
U.S. |
for revealing the behaviour of substances at extremely low temperatures. His research confirmed the third law of thermodynamics, which states that the entropy of ordered solids reaches zero at the absolute zero of temperature. In the course of his low-temperature studies of oxygen, Giauque discovered with Herrick L. Johnston the oxygen isotopes of mass 17 and 18. |
|
1950 |
|
West Germany |
for the discovery and development of diene synthesis. The diene synthesis consists essentially of the linking of a diene, which is a substance containing two alternate double bonds, to a dienophile, which is a compound containing a pair of doubly or triply bonded carbon atoms. The diene and dienophile readily react to form a six-membered ring compound. Similar reactions had been recorded by others, but it was Alder and Diels who provided the first experimental proof of the nature of the reaction and demonstrated its application to the synthesis of a wide range of ring compounds. Diene synthesis can be effected without the use of powerful chemical reagents. It has been used to synthesize such complex molecules as morphine, reserpine, cortisone and other steroids, the insecticides dieldrin and aldrin, and other alkaloids and polymers. |
|
|
West Germany |
|||
1951 |
|
U.S. |
for the discovery of and research on transuranium elements |
|
|
U.S. |
|||
1952 |
|
U.K. |
for the development of partition chromatography. Martin and Synge invented paper partition chromotography in 1944. Partition chromatography depends on the partition, or distribution, of each component of a mixture between two immiscible liquids. One of the liquids is held stationary by strong adsorption on the surface of a finely divided solid while the other flows through the interstices of the solid particles. Any substance that preferentially dissolves in the mobile liquid is more rapidly transported in the direction of flow than is a substance that has greater affinity for the stationary liquid. |
|
|
U.K. |
|||
1953 |
|
West Germany |
for their work on macromolecules; devised a relationship between viscosity and molecular weight |
|
1954 |
|
U.S. |
study of the nature of the chemical bond; introduced hybridization. In order to account for the equivalency of the four bonds around the carbon atom, he introduced the concept of hybrid orbitals, in which electron orbits are moved from their original positions by mutual repulsion. Pauling also recognized the presence of hybrid orbitals in the coordination of ions or groups of ions in a definite geometric arrangement about a central ion. His theory of directed (positive and negative) valence (the capacity of an atom to combine with other atoms) was an outgrowth of his early work, as was the concept of the partial ionic character of covalent bonds--i.e., atoms sharing electrons. His empirical concept of electronegativity, the power of attraction for electrons in a covalent bond, was useful in further clarification of these problems. In the case of compounds whose molecules cannot be represented unambiguously by a single structure, he introduced the concept of resonance hybrids whereby the true structure of the molecule is regarded as an intermediate state between two or more depictable structures. The resonance theory came under heavy but unsuccessful attack in the U.S.S.R. in 1951 when doctrinaire scientists of the Communist Party argued that it conflicted with dialectical materialist principles. |
|
1955 |
|
U.S. |
for the isolation and synthesis of two pituitary hormones: vasopressin, which acts on the muscles of the blood vessels to cause elevation of blood pressure; and oxytocin, the principal agent causing contraction of the uterus and secretion of milk. |
|
1956 |
|
U.K. |
for their work on the kinetics of chemical reactions |
|
Semyonov, Nikolay Nikolayevich |
U.S.S.R. |
|||
1957 |
Todd (of Trumpington), Alexander Robertus Todd, Baron |
U.K. |
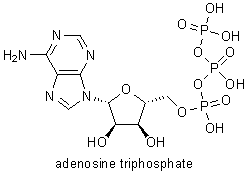
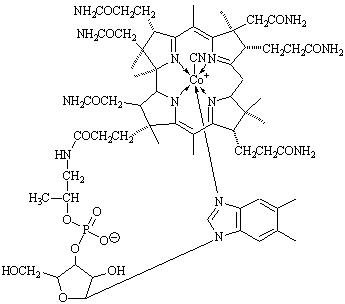
While at Manchester he began work on nucleosides, compounds that form the structural units of nucleic acids (DNA and RNA). In 1949 he synthesized a related substance, adenosine triphosphate (ATP), which is vital to energy utilization in living organisms. He synthesized two other important compounds, flavin adenine dinucleotide (FAD) in 1949 and uridine triphosphate in 1954. In 1955 he elucidated the structure of vitamin B12. |
|
1958 |
|
U.K. |
for the determination of the structure of the insulin molecule |
|
1959 |
|
Czech. |
for the discovery and development of polarography, an instrumental method of chemical analysis used for qualitative and quantitative determinations of reducible or oxidizable substances. Heyrovský's instrument measures the current that flows when a predetermined potential is applied to two electrodes immersed in the solution to be analyzed. |
|
1960 |
|
U.S. |
In 1946 he showed that tritium, the heaviest isotope of hydrogen, was produced by cosmic radiation. The following year he and his students developed the carbon-14 dating technique. This technique is used to date material derived from former living organisms as old as 50,000 years. It measures small amounts of radioactivity from the carbon-14 in organic or carbon-containing materials and is able to identify older objects as those having less radioactivity. |
|
1961 |
|
U.S. |
for the study of chemical steps that take place during photosynthesis. Calvin began his work on photosynthesis in the mid-1940s. For his studies he developed a system of using the radioactive isotope carbon-14 as a tracer element in the green alga Chlorella. By arresting the plant's growth at various stages and measuring the tiny amounts of radioactive compounds present, Calvin was able to identify most of the reactions involved in the intermediate steps of photosynthesis. |
|
1962 |
|
U.K. |
He determined the structure of the muscle protein myoglobin, which stores oxygen and gives it to the muscle cells when needed |
|
|
U.K. |
For his X-ray diffraction analysis of the structure of hemoglobin, the protein that transports oxygen from the lungs to the tissues via blood cells. | ||
1963 |
|
Italy |
for thestructure and synthesis of polymers in the field of plastics. In 1953 Natta began intensive study of macromolecules. Using Ziegler's catalysts, he experimented with the polymerization of propylene and obtained polypropylenes of highly regular molecular structure. The properties--high strength, high melting points--of these polymers soon proved very commercially important. |
|
|
West Germany |
Ziegler's most important discovery was made in 1953. He and a student, E. Holzkamp, set out to repeat a preparation of higher aluminum alkyls by heating ethylene and aluminum triethyl; unexpectedly they obtained a complete conversion of the ethylene monomer (CH2 = CH2) to the dimer, 1-butene (CH3CH2CH = CH2). The explanation was found in the presence of a trace of colloidal nickel derived from the catalyst used previously in the autoclave for hydrogenation experiments. This led to the discovery that substances made by mixing organometallic compounds with compounds of certain heavy metals permitted the fast polymerization of ethylene at atmospheric pressure to a linear polymer of high molecular weight having valuable plastic properties (other processes used high pressure and produced a partly branched polymer). The catalyst derived from aluminum alkyl and titanium tetrachloride proved especially useful. It formed the basis of nearly all later developments in the production of long-chain polymers of hydrocarbons from such olefins as ethylene and butadiene; the resulting products came into widespread use as plastics, fibres, rubbers, and films. | ||
1964 |
Hodgkin, Dorothy Mary Crowfoot |
U.K. |
for determining the structure of biochemical compounds (such as vitamin B12)essential in combating pernicious anemia. It would later take 10 years ( from 1961 to 1971) to synthesize this particular vitamin. |
|
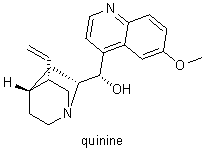
1965 |
|
U.S. |
For the synthesis of compounds such as including quinine (1944), cholesterol and cortisone (1951), and vitamin B12 (1971) |
|
1966 |
|
U.S. |
for work concerning chemical bonds and the electronic structure of molecules. Mulliken began working on his theory of molecular structure in the 1920s. He theoretically systematized the electron states of molecules in terms of molecular orbitals. Departing from the idea that electron orbitals for atoms are static and that atoms combine like building blocks to form molecules, he proposed that, when molecules are formed, the atoms' original electron configurations are changed into an overall molecular configuration. Further extending his theory, he developed (1952) a quantum-mechanical theory of the behaviour of electron orbitals as different atoms merge to form molecules. |
|
1967 |
|
West Germany |
for studies of extremely fast chemical reactions.
|
|
Norrish, Ronald George Wreyford |
U.K. |
Norrish and Porter, who worked together between 1949 and 1965, used the new technique of flash photolysis to study the intermediate stages involved in extremely rapid chemical reactions. In this technique, a gaseous system in a state of equilibrium is subjected to an ultrashort burst of light that causes photochemical reactions in the gas. A second burst of light is then used to detect and record the changes taking place in the gas before equilibrium is reestablished. | ||
|
U.K. |
|||
1968 |
|
U.S. |
He applied the laws of thermodynamics to systems that are not in equilibrium--i.e., to systems in which differences in temperature, pressure, or other factors exist. Onsager also was able to formulate a general mathematical expression about the behaviour of nonreversible chemical processes that has been described as the "fourth law of thermodynamics." |
|
1969 |
|
U.K. |
for research that helped establish conformational analysis (the study of the three-dimensional geometric structure of complex molecules) as an essential part of organic chemistry. |
|
|
Norway |
|||
1970 |
|
Argentina |
for the discovery of sugar nucleotides and their role in the biosynthesis of carbohydrates |
|
1971 |
|
Canada |
research in the structure of molecules. Herzberg's spectroscopic studies not only provided experimental results of prime importance to physical chemistry and quantum mechanics but also helped stimulate a resurgence of investigations into the chemical reactions of gases. He devoted much of his research to diatomic molecules, in particular the most common ones--hydrogen, oxygen, nitrogen, and carbon monoxide. He discovered the spectra of certain free radicals that are intermediate stages in numerous chemical reactions, and he was the first to identify the spectra of certain radicals in interstellar gas. Herzberg also contributed much spectrographic information on the atmospheres of the outer planets and the stars. |
|
1972 |
|
U.S. |
Anfinsen studied how the enzyme ribonuclease breaks down the ribonucleic acid (RNA) present in food. Anfinsen was able to ascertain how the ribonuclease molecule folds to form the characteristic three-dimensional structure that is compatible with its function. |
|
|
U.S. |
|||
|
U.S. |
Moore and Stein pioneered new methods of chromatography for use in analyzing amino acids and small peptides obtained by the hydrolysis of proteins. In 1958 they helped develop the first automatic amino-acid analyzer, a machine that greatly facilitated the analysis of the amino acid sequences of proteins. In 1959 Moore and Stein used the new machine to make the first determination of the complete chemical structure of an enzyme, ribonuclease. | ||
1973 |
|
West Germany |
organometallic chemistry For his identification of a completely new way in which metals and organic substances can combine. After studying the synthetic substance ferrocene, he concluded that it consisted of two five-sided carbon rings with a single iron atom sandwiched between them. |
|
|
U.K. |
Wilkinson went on to synthesize a number of other "sandwich" compounds, or metallocenes, and his researches into this previously unknown type of chemical structure earned him the Nobel Prize. | ||
1974 |
|
U.S. |
for studies of long-chain molecules involved in plastics. His research demonstrated the importance of understanding the sizes and shapes of these flexible molecules in establishing relationships between their chemical structures and their physical properties.. |
|
1975 |
|
U.K. |
for work in stereochemistry of enzyme-catalyzed reactions such as the one involved in the synthesis of cholestrol |
|
|
Switzerland |
for work in stereochemistry of of alkaloids, antibiotics, enzymes, and other natural compounds |
||
1976 |
|
U.S. |
for the structure of boranes. By developing X-ray techniques that later proved useful in many chemical applications, Lipscomb and his associates were able to map the molecular structures of numerous boranes and their derivatives. Boranes are compounds of boron and hydrogen. The stability of boranes could not be explained by traditional concepts of electron bonding, in which each pair of atoms is linked by a pair of electrons, because boranes lacked sufficient electrons. Lipscomb showed how a pair of electrons could be shared by three atoms. His theory successfully served to describe boranes and many other analogous structures. |
|
1977 |
|
Belgium |
for widening the scope of thermodynamics.
|
|
1978 |
|
U.K. |
for the formulation of a theory of energy transfer processes in biological systems. Mitchell studied the mitochondrion, the organelle that produces energy for the cell. ATP is made within the mitochondrion by adding a phosphate group to ADP in a process known as oxidative phosphorylation. Mitchell was able to determine how the different enzymes involved in the conversion of ADP to ATP are distributed within the membranes that partition the interior of the mitochondrion. He showed how these enzymes' arrangement facilitates their use of hydrogen ions as an energy source in the conversion of ADP to ATP |
|
1979 |
|
U.S. |
introduction of compounds of boron and phosphorus in the synthesis of organic substances
Brown's work with borohydrides led to the development of an important new class of inorganic reagents. His discovery of the organoboranes revealed an array of powerful and versatile reagents for organic synthesis. He was also known for studies of reactions involving so-called carbonium ions or carbo-cations. |
|
|
West Germany |
In investigating reactions involving carbanions, negatively charged organic species, Wittig discovered a class of organic phosphorus compounds called ylides that mediate a particular type of reaction that became known as the Wittig reaction. This reaction proved of great value in the synthesis of complex organic compounds such as vitamins A and D2, prostaglandins, and steroids | ||
1980 |
|
U.S. |
first preparation of a recombinant DNA.
|
|
|
U.S. |
for the development of chemical and biological analyses of DNA structure. In the 1970s Gilbert developed a widely used technique of using gel electrophoresis to read the nucleotide sequences of DNA segments. The same method was developed independently by Sanger |
||
|
U.K. |
By 1977 he had elucidated the sequences of nucleotides in the DNA molecule of a bacteriophage (a virus that infects bacteria). This phage, phi-X 174, was the first organism to have its entire nucleotide sequence determined. To achieve this feat, Sanger again developed new laboratory techniques, this time for splitting DNA into fragments whose base sequences can then be determined. | ||
1981 |
|
Japan |
for the orbital symmetry interpretation of chemical reactions. Hoffmann and Woodward discovered that many reactions involving the formation or breaking of rings of atoms take courses that depend on an identifiable symmetry in the mathematical descriptions of the molecular orbitals that undergo the most change. Their theory, expressed in a set of statements now called the Woodward-Hoffmann rules, accounts for the failure of certain cyclic compounds to form from apparently appropriate starting materials, though others are readily produced; it also clarifies the geometric arrangement of the atoms in the products formed when the rings in cyclic compounds are broken. |
|
|
U.S. |
|||
1982 |
|
U.K. |
for investigations of the three-dimensional structure of viruses and other particles that are combinations of nucleic acids and proteins, and for the development of crystallographic electron microscopy. |
|
1983 |
|
U.S.(Canadian born) |
for his extensive research into the properties and reactions of dissolved inorganic substances, particularly oxidation-reduction processes involving the ions of metallic elements.
|
|
1984 |
|
U.S. |
for the development of a method of polypeptide synthesis |
|
1985 |
|
U.S. |
Hauptman and Karle devised mathematical equations to extract phase information from the intensity of spots resulting from the diffraction of X rays deflected off crystals. Their equations made it possible to pinpoint the location of atoms within the crystal's molecules based upon an analysis of the intensity of the spots. |
|
|
U.S. |
|||
1986 |
|
U.S. |
for the development of methods for analyzing basic chemical reactions |
|
|
U.S. |
|||
|
Canada |
|||
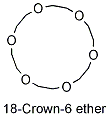
1987 |
|
U.S. |
for the discovery of crown ethers, organic molecules that can solvate metal ions, which normally do not dissolve in organic solvents. |
|
|
|
France |
Formed cryptands from crown ethers by replacing O’s with N’s |
||
|
|
U.S. |
Formed cryptates, which selectively recognized and bound specific guest molecules and ions. |
||
1988 |
|
West Germany |
for the discovery of structure of proteins needed in photosynthesis.
|
|
|
West Germany |
|||
|
West Germany |
|||
1989 |
|
U.S. (born in Canada) |
for the discovery of certain basic properties of RNA. The old belief was that enzymatic activity--the triggering and acceleration of vital chemical reactions within living cells--was the exclusive domain of protein molecules. Altman's and Cech's revolutionary discovery was that RNA, traditionally thought to be simply a passive carrier of genetic codes between different parts of the living cell, could also take on active enzymatic functions. This new knowledge opened up new fields of scientific research and biotechnology and caused scientists to rethink old theories of how cells function. |
|
|
U.S. |
|||
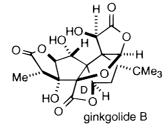
1990 |
|
U.S. |
for the development of retrosynthetic analysis for synthesis of complex molecules. This technique involves breaking down an organic compound into several stages, ensuring that each step is reversible so that the route can eventually be traced backwards. With such an approach, Corey synthesied a wide variety of terpenes and ginkgolide B (used in treating asthma). Link to synthesis. |
|
1991 |
|
Switzerland |
for improvements in nuclear magnetic resonance spectroscopy. In 1966, working with an American colleague, Ernst discovered that the sensitivity of NMR techniques (hitherto limited to analysis of only a few nuclei) could be dramatically increased by replacing the slow, sweeping radio waves traditionally used in NMR spectroscopy with short, intense pulses. His discovery enabled analysis of a great many more types of nuclei and smaller amounts of materials. |
|
1992 |
|
U.S. (born Mtl,Canada) |
for an explanation of how electrons transfer between molecules. In a series of papers published between 1956 and 1965, he investigated the role of surrounding solvent molecules in determining the rate of redox reactions--oxidation and reduction reactions in which the reactants exchange electrons--in solution. Marcus determined that subtle changes occur in the molecular structure of the reactants and the solvent molecules around them; these changes influence the ability of electrons to move between the molecules. He further established that the relationship between the driving force of an electron-transfer reaction and the reaction's rate is described by a parabola. Thus, as more driving force is applied to a reaction, its rate at first increases but then begins to decrease. This insight aroused considerable skepticism until it was confirmed experimentally in the 1980s. |
|
1993 |
|
U.S. |
inventors of techniques for gene study and manipulation. Mullis developed PCR (polymerase chain reaction) in 1983. Earlier methods for obtaining a specific sequence of DNA in quantities sufficient for study were difficult, time-consuming, and expensive. PCR uses four ingredients: the double-stranded DNA segment to be copied, called the template DNA; two oligonucleotide primers (short segments of single-stranded DNA, each of which is complementary to a short sequence on one of the strands of the template DNA); nucleotides, the chemical building blocks that make up DNA; and a polymerase enzyme that copies the template DNA by joining the free nucleotides in the correct order. These ingredients are heated, causing the template DNA to separate into two strands. The mixture is cooled, allowing the primers to attach themselves to the complementary sites on the template strands. The polymerase is then able to begin copying the template strands by adding nucleotides onto the end of the primers, producing two molecules of double-stranded DNA. Repeating this cycle increases the amount of DNA exponentially: some 30 cycles, each lasting only a few minutes, will produce more than a billion copies of the original DNA sequence. Before the advent of Smith's method, the technique biochemical researchers used to create genetic mutations was imprecise and the haphazard approach made it a difficult and time-consuming task. Smith remedied this situation by developing site-directed mutagenesis, a technique that can be used to modify nucleotide sequences at specific, desired locations within a gene. This had made it possible for researchers to determine the role each amino acid plays in protein structure and function. Aside from its value to basic research, site-directed mutagenesis has many applications in medicine, agriculture, and industry. For example, it can be used to produce a protein variant that is more stable, active, or useful than its natural counterpart. |
|
|
Canada |
|||
1994 |
|
U.S. |
development of techniques to study hydrocarbon molecules. Although theoretically recognized for several decades as a common intermediate in many organic reactions, carbocations were unobservable because they were a short-lived, unstable class of compound. Olah was able to successfully disassemble, examine, and then recombine carbocations through the use of superacids and ultracold solvents. |
|
1995 |
|
Netherlands |
explanation of processes that deplete Earth's ozone layer. Rowland and Molina theorized that CFC gases combine with solar radiation and decompose in the stratosphere, releasing atoms of chlorine and chlorine monoxide that are individually able to destroy large numbers of ozone molecules. The chlorine atoms were persistent because they were recycled once an intermediate radical, ClO, reacted with atomic oxygen. Earlier, in 1970 Crutzen had discovered that nonreactive nitrous oxide (N2O), produced naturally by soil bacteria, rises into the stratosphere, where solar energy splits it into two reactive compounds, NO and NO2. These compounds, which remain active for some time, react catalytically with ozone (O3). Mainly because of their influence, production of CFC's was eventually banned. |
|
|
U.S. | |||
|
U.S. | |||
1996 |
|
U.S. |
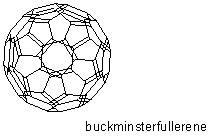
discovery of new carbon compounds called fullerenes, spherical clusters of carbon atoms By crystallizing buckminsterfullerene at high pressures, other chemists created a material that could scratch diamond. |
|
|
U.K. |
|||
|
U.S. |
|||
1997 |
PAUL D. BOYER
|
U.S. |
for their elucidation of the enzymatic mechanism underlying the synthesis of adenosine triphosphate (ATP) |
|
JOHN E. WALKER  |
||||
| JENS C. SKOU
|
Denmark |
for the first discovery of an ion-transporting enzyme, Na+, K+-ATPase. |
||
1998 |
|
U.K. |
for pioneering contributions in developing methods(quantum chemistry) that can be used for theoretical studies of molecular properties and related chemical processes. |
|
| WALTER KOHN
|
U.S.(born Austria) | |||
1999 |
|
U.S. (born Egypt) |
for his studies of the transition states of chemical reactions using femtosecond spectroscopy. |
|
2000 |
|
U.S. |
for the discovery and development of conductive polymers. |
|
ALAN G. MACDIARMID,  |
U.S. (born New Zealand) |
|||
HIDEKI SHIRAKAWA  |
Japan | |||
2001 |
|
U.S. |
for their work on chirally-catalysed oxidation reactions. They developed molecules that can catalyze important reactions so that only one of the two mirror image forms is produced. The catalyst molecule, which itself is chiral, speeds up the reaction without being consumed. Just one of these molecules can produce millions of molecules of the desired mirror image form. |
|
|
U.S. |
|||
| and RYOJI NOYORI,
|
Japan | |||
2002 |
U.S. |
for their development of soft desorption ionization methods for mass spectrometric analyses of biological macromolecules |
||
| KOICHI TANAKA,
|
Japan | |||
2003 |
PETER AGRE |
U.S. |
for advancing knowledge about cellular membrane channels — passageways that control the movement of molecules across cell membranes. More details. |
|
RODERICK MACKINNON  |
U.S. | |||
| 2004 | Aaron Ciechanover |
Israel | ||
Avram Hershko |
Israel | |||
Irwin Rose |
U.S. | |||
2005 |
YVES CHAUVIN |
France |
For developing metathesis reactions in which double bonds are broken and made
between carbon atoms in ways that cause atom groups to change places. This happens with the
assistance of special
catalyst molecules. Metathesis can be compared to a dance in which the couples change partners. |
|
Grubbs |
U.S. | |||
Richard Schrock |
U.S. | |||
| 2006 | Roger
Kornberg |
U.S. | For an atomic-level explanation of how DNA is copied into messenger RNA (transcription). His work may some day help stem-cell research. | |
| 2007 | Gerhard Ertl
|
Germany | Gerhard Ertl
is known for determining the detailed molecular mechanisms of the
catalytic synthesis of ammonia over iron (Haber
Bosch process) and the catalytic oxidation of carbon monoxide over palladium (catalytic
converter). During his research he discovered the important
phenomenon of oscillatory reactions on platinum surfaces and, using
photoelectron microscopy, was able to image for the first time, the
oscillating changes in surface structure and coverage that occur
during the reaction.
He always used new observation techniques like low energy electron diffraction (LEED) at the beginning of his career, later ultraviolet photoelectron spectroscopy (UPS) and scanning tunneling microscope (STM) yielding ground breaking results. (directly from Wikipedia) |
|
| 2008 |
|
U.S. | The three
scientists were awarded the prize for their work on the green
fluorescent protein (GFP), which is visible under blue and
ultraviolet light.
GFP, first isolated from jellyfish, attaches itself to regulatory proteins in the cell and allows them to be monitored The GFP gene can now be cloned and produced in different colours to track different proteins simultaneously. GFP has been used to detect pollutants in the environment, in glow-in-the-dark toys, and to study the growth of tumour cells. For more details see http://nobelprize.org/nobel_prizes/chemistry/laureates/2008/info.pdf |
|
|
U.S. | |||
|
U.S | |||
| 2009 | Venkatraman Ramakrishnan |
U.K |
For studies of the structure and function of the ribosome
Using Xray crystallography, the three recipients generated 3D models to show how different antibiotics bind to the ribosome. What a nice example of intermolecular bonding! These models are now used to develop new antibiotics. 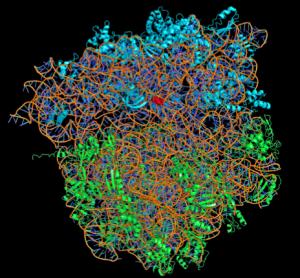 http://www.sciencedaily.com: An X-ray structure of a bacterium ribosome. The rRNA molecules are colored orange, the proteins of the small subunit are blue and the proteins of the large subunit are green. An antibiotic molecule (red) is bound to the small subunit. Scientists study these structures in order to design new and more effective antibiotics. (Credit: Image courtesy of Nobel Foundation)
|
|
Thomas A. Steitz
|
U.S. | |||
Ada E. Yonath
|
Israel |
| 2010 | U.S. |
. Directly from: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2010/press.html
Organic chemistry has developed into an art form where scientists produce marvelous chemical creations in their test tubes. Mankind benefits from this in the form of medicines, ever-more precise electronics and advanced technological materials. The Nobel Prize in Chemistry 2010 awards one of the most sophisticated tools available to chemists today. This year's Nobel Prize in Chemistry is awarded to Richard F. Heck, Ei-ichi Negishi and Akira Suzuki for the development of palladium-catalyzed cross coupling. This chemical tool has vastly improved the possibilities for chemists to create sophisticated chemicals, for example carbon-based molecules as complex as those created by nature itself. Carbon-based (organic) chemistry is the basis of life and is responsible for numerous fascinating natural phenomena: colour in flowers, snake poison and bacteria killing substances such as penicillin. Organic chemistry has allowed man to build on nature's chemistry; making use of carbon’s ability to provide a stable skeleton for functional molecules. This has given mankind new medicines and revolutionary materials such as plastics. In order to create these complex chemicals, chemists need to be able to join carbon atoms together. However, carbon is stable and carbon atoms do not easily react with one another. The first methods used by chemists to bind carbon atoms together were therefore based upon various techniques for rendering carbon more reactive. Such methods worked when creating simple molecules, but when synthesizing more complex molecules chemists ended up with too many unwanted by-products in their test tubes. Palladium-catalyzed cross coupling solved that problem and provided chemists with a more precise and efficient tool to work with. In the Heck reaction, Negishi reaction and Suzuki reaction, carbon atoms meet on a palladium atom, whereupon their proximity to one another kick-starts the chemical reaction. Palladium-catalyzed cross coupling is used in research worldwide, as well as in the commercial production of for example pharmaceuticals and molecules used in the electronics industry. |
|
|
Japan | ||
|
Japan |
| 2011 |
|
Israel |
. Directly from: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2011/press.html
In quasicrystals, we find the fascinating mosaics of the Arabic world reproduced at the level of atoms: regular patterns that never repeat themselves. However, the configuration found in quasicrystals was considered impossible, and Dan Shechtman had to fight a fierce battle against established science. The Nobel Prize in Chemistry 2011 has fundamentally altered how chemists conceive of solid matter. On the morning of 8 April 1982, an image counter to the laws of nature appeared in Dan Shechtman's electron microscope. In all solid matter, atoms were believed to be packed inside crystals in symmetrical patterns that were repeated periodically over and over again. For scientists, this repetition was required in order to obtain a crystal. Shechtman's image, however, showed that the atoms in his crystal were packed in a pattern that could not be repeated. Such a pattern was considered just as impossible as creating a football using only six-cornered polygons, when a sphere needs both five- and six-cornered polygons. His discovery was extremely controversial. In the course of defending his findings, he was asked to leave his research group. However, his battle eventually forced scientists to reconsider their conception of the very nature of matter. Aperiodic mosaics, such as those found in the medieval Islamic mosaics of the Alhambra Palace in Spain and the Darb-i Imam Shrine in Iran, have helped scientists understand what quasicrystals look like at the atomic level. In those mosaics, as in quasicrystals, the patterns are regular - they follow mathematical rules - but they never repeat themselves. When scientists describe Shechtman's quasicrystals, they use a concept that comes from mathematics and art: the golden ratio. This number had already caught the interest of mathematicians in Ancient Greece, as it often appeared in geometry. In quasicrystals, for instance, the ratio of various distances between atoms is related to the golden mean. Following Shechtman's discovery, scientists have produced other kinds of quasicrystals in the lab and discovered naturally occurring quasicrystals in mineral samples from a Russian river. A Swedish company has also found quasicrystals in a certain form of steel, where the crystals reinforce the material like armor. Scientists are currently experimenting with using quasicrystals in different products such as frying pans and diesel engines. |
| 2012 | U.S. |
. Directly from: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2012/press.html
Your body is a fine-tuned system of interactions between billions of cells. Each cell has tiny receptors that enable it to sense its environment, so it can adapt to new situations. Robert Lefkowitz andBrian Kobilka are awarded the 2012 Nobel Prize in Chemistry for groundbreaking discoveries that reveal the inner workings of an important family of such receptors: G-protein–coupled receptors. For a long time, it remained a mystery how cells could sense their environment. Scientists knew that hormones such as adrenalin had powerful effects: increasing blood pressure and making the heart beat faster. They suspected that cell surfaces contained some kind of recipient for hormones. But what these receptors actually consisted of and how they worked remained obscured for most of the 20th Century. Lefkowitz started to use radioactivity in 1968 in order to trace cells' receptors. He attached an iodine isotope to various hormones, and thanks to the radiation, he managed to unveil several receptors, among those a receptor for adrenalin: β-adrenergic receptor. His team of researchers extracted the receptor from its hiding place in the cell wall and gained an initial understanding of how it works. The team achieved its next big step during the 1980s. The newly recruited Kobilka accepted the challenge to isolate the gene that codes for the β-adrenergic receptor from the gigantic human genome. His creative approach allowed him to attain his goal. When the researchers analyzed the gene, they discovered that the receptor was similar to one in the eye that captures light. They realized that there is a whole family of receptors that look alike and function in the same manner. Today this family is referred to as G-protein–coupled receptors. About a thousand genes code for such receptors, for example, for light, flavour, odour, adrenalin, histamine, dopamine and serotonin. About half of all medications achieve their effect through G-protein–coupled receptors. The studies by Lefkowitz and Kobilka are crucial for understanding how G-protein–coupled receptors function. Furthermore, in 2011, Kobilka achieved another break-through; he and his research team captured an image of the β-adrenergic receptor at the exact moment that it is activated by a hormone and sends a signal into the cell. This image is a molecular masterpiece – the result of decades of research. |
|
|
U.S. |
| 2013 | Austria/U.S |
. Directly from: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2013/press.html
Chemists used to create models of molecules using plastic balls and sticks. Today, the modelling is carried out in computers. In the 1970s,Martin Karplus, Michael Levitt and Arieh Warshel laid the foundation for the powerful programs that are used to understand and predict chemical processes. Computer models mirroring real life have become crucial for most advances made in chemistry today. Chemical reactions occur at lightning speed. In a fraction of a millisecond, electrons jump from one atomic to the other. Classical chemistry has a hard time keeping up; it is virtually impossible to experimentally map every little step in a chemical process. Aided by the methods now awarded with the Nobel Prize in Chemistry, scientists let computers unveil chemical processes, such as a catalyst’s purification of exhaust fumes or the photosynthesis in green leaves. The work of Karplus, Levitt and Warshel is ground-breaking in that they managed to make Newton’s classical physics work side-by-side with the fundamentally different quantum physics. Previously, chemists had to choose to use either or. The strength of classical physics was that calculations were simple and could be used to model really large molecules. Its weakness, it offered no way to simulate chemical reactions. For that purpose, chemists instead had to use quantum physics. But such calculations required enormous computing power and could therefore only be carried out for small molecules. This year’s Nobel Laureates in chemistry took the best from both worlds and devised methods that use both classical and quantum physics. For instance, in simulations of how a drug couples to its target protein in the body, the computer performs quantum theoretical calculations on those atoms in the target protein that interact with the drug. The rest of the large protein is simulated using less demanding classical physics. Today the computer is just as important a tool for chemists as the test tube. Simulations are so realistic that they predict the outcome of traditional experiments. |
|
|
S. Africaa/U.S. | ||
|
Israel/U.S |
| 2014 | U.S. |
. Directly from: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2013/press.html
For a long time optical microscopy was held back by a presumed limitation: that it would never obtain a better resolution than half the wavelength of light. Helped by fluorescent molecules the Nobel Laureates in Chemistry 2014 ingeniously circumvented this limitation. Their ground-breaking work has brought optical microscopy into the nanodimension. In what has become known as nanoscopy, scientists visualize the pathways of individual molecules inside living cells. They can see how molecules create synapses between nerve cells in the brain; they can track proteins involved in Parkinson's, Alzheimer's and Huntington's diseases as they aggregate; they follow individual proteins in fertilized eggs as these divide into embryos. It was all but obvious that scientists should ever be able to study living cells in the tiniest molecular detail. In 1873, the microscopist Ernst Abbe stipulated a physical limit for the maximum resolution of traditional optical microscopy: it could never become better than 0.2 micrometres. Eric Betzig, Stefan W. Helland William E. Moerner are awarded the Nobel Prize in Chemistry 2014 for having bypassed this limit. Due to their achievements the optical microscope can now peer into the nanoworld. Two separate principles are rewarded. One enables the method stimulated emission depletion (STED) microscopy, developed by Stefan Hell in 2000. Two laser beams are utilized; one stimulates fluorescent molecules to glow, another cancels out all fluorescence except for that in a nanometre-sized volume. Scanning over the sample, nanometre for nanometre, yields an image with a resolution better than Abbe's stipulated limit. Eric Betzig and William Moerner, working separately, laid the foundation for the second method, single-molecule microscopy. The method relies upon the possibility to turn the fluorescence of individual molecules on and off. Scientists image the same area multiple times, letting just a few interspersed molecules glow each time. Superimposing these images yields a dense super-image resolved at the nanolevel. In 2006 Eric Betzig utilized this method for the first time. Today, nanoscopy is used world-wide and new knowledge of greatest benefit to mankind is produced on a daily basis. |
|
|
.Germany | ||
|
U.S. |
| 2015 |
|
.
|
|
|
|||
|





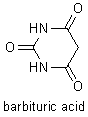



































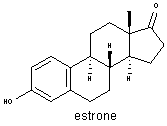 In 1931 he isolated and identified androsterone, a male
sex hormone, and in 1934, the hormone progesterone, which plays an
important part in the female reproductive cycle. It was now clear
that sex hormones are closely related to steroids, and after Ruzicka
showed that cholesterol could be transformed into androsterone, he
and Butenandt were able to synthesize both progesterone and the male
hormone testosterone. Butenandt's investigations made possible the
eventual synthesis of cortisone and other steroids and led to the
development of birth control pills.
In 1931 he isolated and identified androsterone, a male
sex hormone, and in 1934, the hormone progesterone, which plays an
important part in the female reproductive cycle. It was now clear
that sex hormones are closely related to steroids, and after Ruzicka
showed that cholesterol could be transformed into androsterone, he
and Butenandt were able to synthesize both progesterone and the male
hormone testosterone. Butenandt's investigations made possible the
eventual synthesis of cortisone and other steroids and led to the
development of birth control pills.
 Before this discovery, rings
with more than eight atoms had been unknown and indeed had
been believed to be too unstable to exist. Ruzicka's
discovery greatly expanded research on these compounds. He
also showed that the carbon skeletons of terpenes and many
other large organic molecules are constructed from multiple
units of isoprene. In the mid-1930s Ruzicka discovered the
molecular structure of several male sex hormones, notably
testosterone and androsterone, and subsequently synthesized
them.
Before this discovery, rings
with more than eight atoms had been unknown and indeed had
been believed to be too unstable to exist. Ruzicka's
discovery greatly expanded research on these compounds. He
also showed that the carbon skeletons of terpenes and many
other large organic molecules are constructed from multiple
units of isoprene. In the mid-1930s Ruzicka discovered the
molecular structure of several male sex hormones, notably
testosterone and androsterone, and subsequently synthesized
them.
































































































 This
fastens to the protein to be destroyed,
accompanies it to the proteasome where it is
recognised as the key in a lock, and signals
that a protein is on the way for disassembly.
Shortly before the protein is squeezed into the
proteasome, its ubiquitin label is disconnected
for re-use.
This
fastens to the protein to be destroyed,
accompanies it to the proteasome where it is
recognised as the key in a lock, and signals
that a protein is on the way for disassembly.
Shortly before the protein is squeezed into the
proteasome, its ubiquitin label is disconnected
for re-use.