1. Here’s the fast way, as shown in class. But from # 2 onwards; I’ll only show it, step by step using proportions instead of the ratio.
a. 3
moles of NH3 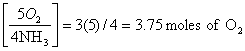
or
a. equation shows: 5 O2 4NH3, so
![]()
4x = 15
x = 3.75 moles of O2
b.
3 moles of O2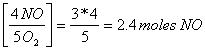
![]()
![]()
Or
![]()
x = 12/5 = 2.4 moles of NO
2.4 moles of NO (14 +16 g/mole) =72 g
c.
2.8 g NO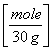 = 0.0933 moles NO
= 0.0933 moles NO
0.0933 moles NO 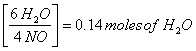
or 2.8 g NO /(14 +16 g/mole) =0.0933 moles NO
![]()
x = 0.14 moles of water
d.
90 g H2O 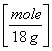 = 5 moles H2O
= 5 moles H2O
5 moles H2O 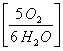 =
= ![]() = 4.166 moles O2
= 4.166 moles O2
4.166 moles O2![]()
or 90 g
H2O / (18 g/mole) = 5.0 moles H2O
![]()
x = 25/6 = 4.2 moles O2
4.2 moles O2(32 g/mole) = 133 g
2. a. answer = 8 moles
b. H2 + Cu2S à 2 Cu + H2S
1g H2/(2g/mole) = 0.5 mole H2
From ratio, twice as many moles of Cu will be produced:
0.5 (2) = 1.0 moles = 63.5 g
3. a. Given: C6H14 +
9.5 O2 à 6 CO2 + 7
H2O + 3500 kJ
a. How
much heat in kJ will be released if only 0.34 moles of C6H14
react?(treat kJ like moles)
b. How
many moles of CO2 will escape if 4.5 moles of oxygen react?
Answer Since kJ are part of the equation, you can treat them like moles.
0.34 moles of C6H14
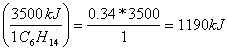
b.
x = 2.84 moles CO2
4. 16 KClO3 +
3 P4S3 à 6 P2O5 + 16 KCl + 9 SO2
a. How
many grams of sulfur dioxide escape each time 0.0010 moles of KClO3
react?
0.0010 moles of KClO3 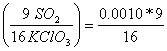 = 0.0005625 moles SO2
= 0.0005625 moles SO2
0.0005625 moles SO2*(64 g/mole) = 0.036 g SO2
b. 4.4 g of P4S3
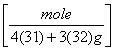 = 0.02 moles of P4S3.
= 0.02 moles of P4S3.
Then apply the ratio and you will obtain 0.06 moles of SO2.
c. 12.2 g KClO3/ (39+ 35.3 + 48 g/mole) = 0.10 moles of KClO3
Apply the ratio: = 0.10 moles of KCl
Then convert to grams: 0.10 (39+35.5) = 7.5 g KCl
5. a. equation reveals that 4 KNO3 react with 7 moles of C, so
Apply the ratio and you will get 3.5 moles of C
3.5 moles of C = 3.5 * 12 = 42 g of C
b. 1010 g KNO3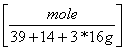 = 10 moles KNO3
= 10 moles KNO3
from
the equation we get the ratio,( remember we are comparing KNO3 to
both CO and CO2
; x = 30/4 = 7.5 moles of CO and 7.5 moles of CO2.
7.5 moles of CO2.![]() = 330 g of CO2.
= 330 g of CO2.
7.5
moles of CO2.![]() = 210 g of CO
= 210 g of CO
Total = 330 + 210 = 540 g.
c. 4.4 g = 0.10 moles of CO2.
From the ratio, 0.10/3 = 0.033 moles of S
6. The question was: Vodka is 40% alcohol by volume. Alcohol's density is 0.7893g/mL. What's the minimum mass of H2CrO4 and HCl needed to destroy the alcohol in 2.0 L of vodka?
3 C2H6O
+ 4 H2CrO4 + 12 HCl àC2H4O2
+ 4 CrCl3 + 13 H2O
Vodka is 40%alcohol, so: 0.40 ( 2.0 L) = 0.80 L of alcohol = 800 mL
![]()
![]()
![]()
Apply the ratio from the equation:
![]()
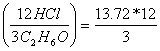
= 54.88 moles of HCl
= 54.88 moles HCl (36.5g/mole) = 2003 g HCl
If they had asked for H2CrO4.
Repeat the procedure.
Start with ![]() . Apply the ratio of 4/3
. Apply the ratio of 4/3
18.3 moles of H2CrO4
18.3
moles H2CrO4 ([2 + 52+64]g /mole) = 2159 g H2CrO4.