Phys Sc 416/36
Pretest 1.1
1. Match the following possible answers with the correct definition.
Possible answers energy, homogeneous, heterogeneous, solution, pure substance, element, compound, matter,
Definitions
a. A substance with only one phase; if it has the same composition throughout it is homogeneous
b. A substance containing only one kind of substance is __pure______________
c. A homogeneous mixture is a _____solution______
d. This contains two or more chemically bonded elements.____compound_______
e. It is the capacity to do work and cannot be weighed____energy____
f. It has a variable composition_____heterogeneous_______
g. It consists of only one type of atom, so its symbol only contains one capital letter___element___
2. When solid iodine is heated, purple crystals turn into a purple gas. Both the crystals and the gas turn white paper brown.
a. Have we described a physical change? Or a chemical change? A physical change
b. Give two reasons to support your answer.
- no colour change
- chemical properties have not changed(same reaction with paper)
3. When 216 grams of a red powder are heated, a gas seems to escape and 200 grams of a silvery liquid remain. The red powder did not react with chlorine; the liquid does.
a. Have we described a physical change? Or a chemical change? chemical change
b. Give 3 reasons to support your answer.
- Mass decreases(216 vs 200)
- Colour change(red to silvery)
- Different chemical properties(original did not react with chlorine; product does)
c. Which of the three substances (powder, gas or liquid) is definitely a compound?
Red powder.
4. The following are properties of oxygen.
- It causes iron to rust.
2. It liquifies at -183o C..
3. It forms water when hydrogen is lit in its presence.
4. It causes a glowing splint to burst into flames.
5. It does not render limewater cloudy.
6. It is released when plants split water molecules during photosynthesis.
a. From the above six properties, which are physical? Only #2
b. Which of the six are characteristic chemical properties? 3,4,and 6
5. When testing for gases, which ones do we commonly hold right-side up, and why?

CO2 and O2, both of which are denser than air.
6. Complete the following to show a chemical change.
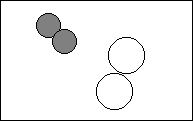 |
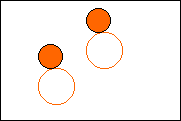 |
||||
7. Give two group characteristic properties of alcohols.
- Flammable
- Have a peculiar smell
8. Match the lettered unknowns with the following four gases:
CO2 O2 H2 H2O (steam)
a. Unknown A: it reacts with cobalt chloride paper to produce a pink colour; no reaction with limewater. H2O
b. Unknown B: it extinguishes a flame; no sound heard CO2
c. Unknown C: no reaction with limewater; candle's flame is bigger in this gas than in air. O2
d. Unknown D: reacts with oxygen H2
9. a. How many atoms are there in 2 molecules of water? 2 X 3 = 6
b. How many atoms of carbon(C) are in Al2(CO3)2 ? 2 How many O's? 2 X 3 = 6
10. List two ways of proving that when magnesium produces a white flash of light, it is undergoing a chemical change.
· Ash, unlike Mg, doesn't burn;
- ash, unlike Mg, doesn't react with acid.
** Also review density problem in notes.