 Phys Sc 430
Phys Sc 430
Pre Test 2.3 Solutions
1.
Identify the solute and solvent in the following
mixtures: (0.5 marks each)
|
Mixture |
Solvent |
Solute(s) |
|
5 g of salt in 100 mL of water |
water |
salt |
|
milk |
water |
Protein, calcium |
|
air |
nitrogen |
Oxygen,argon,carbon dioxide, water |
|
A 4 g/L aqueous solution of NaOH |
water |
NaOH |
2.
a. Two
ions are in solution and are surrounded by water. Figure out which circle is
the positive ion .
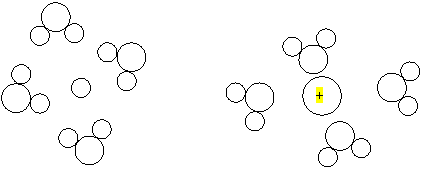
 b. How many water molecules are drawn in the above diagram?___8____
b. How many water molecules are drawn in the above diagram?___8____
- -
3.
![]() Here
is a crystal of KF as it is about to dissolve in water. (1 mark each)
Here
is a crystal of KF as it is about to dissolve in water. (1 mark each)
 Draw what it will look like
after the water dissolves it.
Draw what it will look like
after the water dissolves it.
4. Calculate the mass percent of the following solutions.
(Show work for full marks; (2 marks each))
a. 0.20 g of salt dissolved in 2.5 g of water.
0.20/(0.20 + 2.5)*100% = 7.4%
5. How many grams of RbOH are needed to make 300mL of a 3.0 % solution? Assume a density of 1 g/ml for the solution.
(Show work for full marks) (2 marks )
300g(0.030)
= 9.0 g of RbOH
6. How do you prepare 200 mL of a 3 g/L solution? Show all calculations and all steps used in the laboratory.
Mass = CV = 3g/L(0.200 L) = 0.6
g.
Weigh 0.6 g of solute.
Dissolve in a beaker containing less then 200 mL.
Transfer to a 200 mL volumetric
flask.
Add water to the white mark and mix.
7. Express the concentration in g/L
and in moles/L
0.032 g of O2 are dissolved in 400 mL of
solution.
a. 0.032g/0.400
L = 0.08g/L
b. 0.032g/32(g/mole) = 1.0X 10-3
moles
1.0 X 10-3 moles/ 0.400 L =0.0025 moles/L
8. A student prepares two solutions of
Love Potion #9.
Which is the more concentrated solution? Why?
![]()
![]() = solvent =
solute
= solvent =
solute
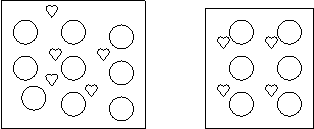
The one on the right is more concentrated because 4 hearts/10 total > 5 hearts/15 total
9. Explain how you would prepare 30 ml of a 2.0 M HCl solution from 1.0 L of 12.0 M solution. Include calculations and a procedure.
C1V1 = C2V2
12 V1= 2(0.030)
V1 = 0.005 L
Pipet 5 ml from the 2.0 M HCl solution
Transfer to a 1.0 L flask.
Add water to the mark and mix.
10. You only have a 25 ml pipette. What size of volumetric flask will you have to use in order to dilute a 2.0 M solution down to 0.25 M?
C1V1 = C2V2
2(0.025) = 0.25 V2
V2 = 0.200L
So you need a 200 mL flask
11. Classify as electrolyte (E), non-electrolyte (N), or “can be either one”(C).
a. a solution that can speed up the rusting of your automobile_E__
b. a solution that would make a good windshield wiper because it not corrosive and because it lowers the freezing point of water_N_
c. ocean water_E_
d. a solution consisting of a compound formed by reacting sulfur and a transition metal_E___
e. a solution whose anions and cations migrate towards anodes and cathode_E__
12. What happens to magnesium ion as electricity is forced into a solution containing such an ion? Include an equation.
Mg+2 + 2eàMg
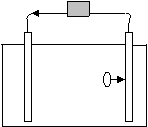
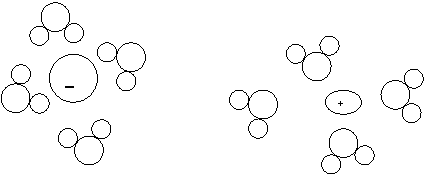
13.
a. In the above diagram, only one ion is shown migrating. What is its charge if the arrows stemming from the gray box represent the flow of electricity?
Negative
b. Justify your answer.
Electrons are flowing towards the right. Since electrons are negative, the opposite side will have a positive charge, which will attract negative ions from the solution.
14. What will happen if the following electrolytes are mixed?
Au(NO3)2 and KBr Write a balanced reaction, knowing that the products are formed by switching metal ions among the reactants.
Au(NO3)2(aq) + 2 KBr(aq) à 2 KNO3(aq) + AuBr2(s)
15. Complete and balance the following ionic equations:
a.
Bi(NO3)2(s)
àBi+2 (aq)+ 2NO3-1(aq)
b.
CaCl2(s) àCa+2(aq) + 2Cl-1(aq)
c. KMnO4(s) à K+1(aq) + MnO4-1(aq)