Movies About Rates
The first movie involves a precipitation reaction. These reactions are extremely quick because no bonds
have to be broken to form a precipitate. The ions are already in solution, and they just attract each other and
form an insoluble product. The equation is as follows:
Ba+2(aq) + 2 Cl-(aq) + 2 Na+1(aq) + SO4-2(aq)
-->2NaCl(aq) + BaSO4(s)
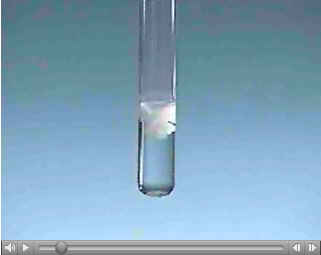
Precipitation Movie
The second movie is an example of a reaction with a very low activation energy. They use a feather to trigger the
decomposition of nitrogen tri-iodide. Many years ago my footsteps set off a sample of the short-fused stuff,
and it exploded in my face.The equation is as follows:
2 NI3(s) --> N2(g) + 3 I2(g)

Low Ae Movie


