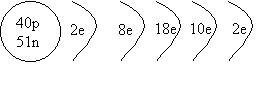Modern Model(continued)
Shell Diagrams ( In Quebec, known as Bohr-Rutherford Diagrams)
The purpose of these diagrams
is to show the nucleus with its correct number of protons and neutrons, but also,
more importantly, to give a more detailed view of electrons, one that will help
explain the chemical properties of elements.
Rules for the First Twenty Elements.
1. When placing electrons in
shells you have to fill, if possible the innermost shell before beginning to
fill the next shell.
2. The maximum number of electrons in the
first shell is 2, which is the number of elements in the first horizontal row
of the periodic table.
3. The maximum number of electrons in the
second shell is 8, which is the number of elements in the second horizontal row
of the periodic table.
4. The maximum number of electrons in the
third shell is 8, which is the number of elements in the third horizontal row
of the periodic table.
Examples:
1. Draw a shell diagram for 7Li.
ANSWER: Lithium 7
has 3 protons and 4 neutrons, and because it's neutral, it has 3 electrons.
We first fill the first shell with 2
electrons( abbreviated 2e): rule #1. The remaining electron has to go into the
third shell.
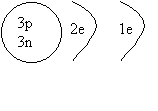
2. Draw a shell diagram
for 40Ca.
ANSWER: Calcium 40 has 20 protons and 20
neutrons, and because it's neutral, it has 20 electrons.
We first fill the first shell with 2
electrons (rule #1) The second shell can hold 8 more, so a total of 10 electrons
have been placed, so far. (rule #2) The
third shell can hold 8 more ( for the first 20 elements; rule # 3), so the last
two electrons end up in the fourth shell.
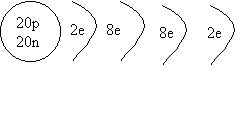
Rules for the Elements # 21-54. Not that important in this course.
1. When placing electrons in
shells you have to fill, if possible the innermost shell before beginning to
fill the next shell.
2. The maximum number of electrons in the
first shell is 2, which is the number of elements in the first horizontal row
of the periodic table.
3. The maximum number of electrons in the
second shell is 8, which is the number of elements in the second horizontal row
of the periodic table.
4. The maximum number of electrons in the
third shell is 18. However before placing more than 8 electrons in this
shell, you have to place two electrons in the next shell, and then come back to
fill the third shell.
5. The maximum number of electrons in the
fourth shell is 18. However before placing more than 8 electrons in this
shell, you have to place two electrons in the next shell, and then come back to
fill the fourth shell.
Example:
1. Draw a shell diagram for 91Zr.
ANSWER: Zirocnium 91
has 40 protons and 51 neutrons, and because it's neutral, it has 40 electrons.
.