Solutions to Review #1
- a.
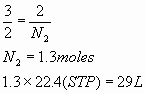
b.
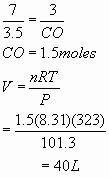
- 4 mL
= 0.004 L =0.004/22.4 =0.000178 moles of C2H6O
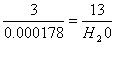
H2O =
0.0007738 moles X 18g/mole = 0.014 g
- A solidís molecules only vibrate.
But if the solid absorbs heat from its surroundings, the molecules vibrate
faster. In a solid that sublimates, the molecules overcome intermolecular
attractions and they begin to both rotate and translate. At this stage we
have a gas.
- No. Temperature depends on the
average kinetic energy of molecules. So although the bulk of molecules
have more or less the average speed, there are extremes that move either
slower or faster. Also, at the same temperature, molecules of a lower
molar mass will, on the average, move faster than those of a higher molar
mass. Remember K.E. = 0.5 mv2.
- P2=350/300P1.
Why? Because if volume and moles are constant, then P1/T1
= P2/T2 from combined gas law. For ideal gases,
pressure is directly proportional to absolute temperature (Kelvin) if
volume remains constant.
6.
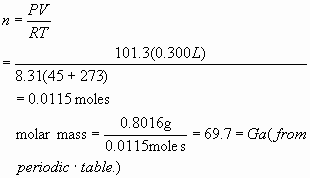
Copyright ©2010
Created:April/6/1996; Updated: Jan/05/2010