Vitamin C
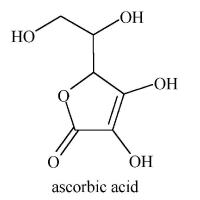

from http://micro.magnet.fsu.edu/micro/gallery.html


When fruits are cooked, much of their vitamin C is oxidized. Ascorbic acid is only stable if its crystals are pure, dry and kept in darkness. Exposed to light and oxygen, ascorbic acid yields electrons and is thus destroyed. In vivo ( in living things), large doses of vitamin A can cause a deficiency of vitamin C.
Vitamin C also interacts with iron, but in a positive way. If a meal contains 25 to 75 mg of ascorbic acid, the absorption of iron is increased.
Needless to say, synthetic vitamin C is chemically identical to the vitamin naturally found in fruits and vegetables. Thus supplements are equally effective in preventing scurvy and in acting as a catalyst for the previously mentioned physiological processes. These facts, however, do not exclude the possibility that other compounds found in fruit and not in supplements help accentuate some of Vitamin C' s functions. In Schwartz's March 7 1999 column for the Montreal Gazette, he cites a Cornell study ( no exact reference given , unfortunately )which clearly showed that vitamin C as an integral part of fruit juices was more effective in reducing the formation of carcinogenic nitrosamines in the body than when taken as a supplement. Chlorogenic acid ( C16H18O9 ), supposedly boosts vitamin C activity.
The Merck Index describes chlorogenic acid as an important factor in plant metabolism. 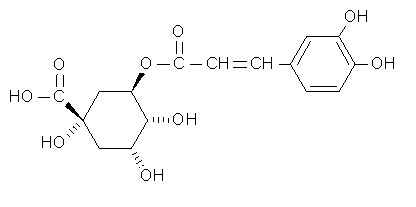 Chlorogenic acid and two of its isomers occur in leaves, fruits and other tissues of dicotyledenous plants (Most commonly eaten fruit are dicots). Like vitamin C, it is a reducing agent . It has long been known as the compound that causes potatoes to blacken when they are cut and exposed to air. (Chem. & Ind. (London) 1958,627) So it is not surprising that it too would attack electron-seeking free radicals. The Vitamin C-chlorogenic acid teamwork reminds us of how numerous carotenoids assist Vitamin A in its physiological role.
Chlorogenic acid and two of its isomers occur in leaves, fruits and other tissues of dicotyledenous plants (Most commonly eaten fruit are dicots). Like vitamin C, it is a reducing agent . It has long been known as the compound that causes potatoes to blacken when they are cut and exposed to air. (Chem. & Ind. (London) 1958,627) So it is not surprising that it too would attack electron-seeking free radicals. The Vitamin C-chlorogenic acid teamwork reminds us of how numerous carotenoids assist Vitamin A in its physiological role.
Copyright ©2008
|
|
|
|