
| Main Pages |
| Home |
| Chem Page |
| Phys Sc Page |
| Monthly Puzzles |

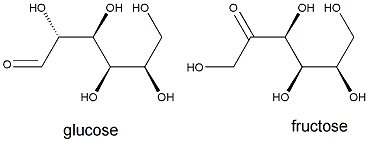
Joseph Louis Gay-Lussac, the same scientist whose results on combining gas volumes were explained by Avogadro, discovered the overall reaction that converts glucose to alcohol and carbon dioxide:
C6H12O6 -->2 CO2+ 2C2H5OH
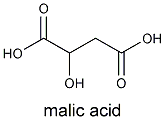
Pasteur realized that yeast was responsible for the conversion of glucose to alcohol. By adding yeast to a simple sugar solution, he showed that alcohol could be formed. He also revealed that the metabolism of yeast was pH -dependent; the acid-level played a role in determining wine's properties. One of grapes' two principal acids, tartaric acid (malic acid is the other), led to Pasteur's discovery of enantiomers, molecules that are mirror images of one another. (The original Pasteur experiment, however, has been difficult to replicate.)
Low acidity (high pH), common in grapes that are too sweet because they were grown in excessively warm climates, lowers the amount of subtle flavours in the grapes and consequently in the wine. As mentioned before, there is an optimum pH for fermentation as well. Prior to fermentation, pH is measured either by titration or more conveniently with a pH meter, and if it is too high, tartaric acid is added.
| Component |
Percent Range |
|---|---|
 |
70-80 |
 |
18-25 |
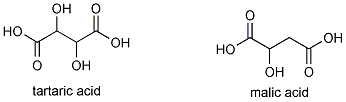  |
0.3-1.5 |
| 20 amino acids
|
0.7 |
| Vitamin C
|
11 mg per 100 g |
|
|
73 IU per 100 g |
| potassium, K+ | 185 mg per 100 g |
| esters
|
less than 0.1% |
| 13 anthocyanins
|
less than 0.1% |
After the grapes are crushed (the stems are mechanically separated), the blend of pulp, skin and seeds are transfered to a vat. (For white wine only the pulp is used). Here SO2 is introduced to kill wild yeasts. These are too varied in composition, leading to a competition amongst themselves that causes fermentation to stop prematurely. They are replaced with a pure culture, usually of Saccharomyces cerevisiae .In addition,SO2, inhibits enzymes that oxidize phenolic compounds responsible for discolouring wine.
Fermentation is an exothermic process ( it releases heat). But in wine-making, the temperature cannot exceed 85 F = 29.4 C for red wines or 60 F = 15.3 C for white wines), otherwise the growth of yeast cells will stop. Moreover, a lower temperature is desirable because it increases the production of esters, other aromatic compunds and alcohol itself. This makes the wine easier to clear and less susceptible to bacterial infection.
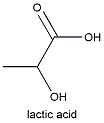
To clarify the wine, the fermented juice is transfered into a settling vat, or if made on a smaller scale, into a demijohn. In these, suspended yeast cells, cream of tartar and particles of skin and pulp settle to the bottom of the container. As the yeast cells break down within the precipitate, they stimulate the growth of Lactobacillus bacteria that convert the wine's malic acid into lactic acid. This process is especially important in wines made from highly acidic grapes because lactic acid is a weaker acid than malic acid. (Bacteria decarboxylate malic acid , thus removing the acidic carboxyl group), so it mellows the wine's taste.
After the demijohn stage, the wine is repeatedly racked to leave behind less and less precipitate. During the repeated pourings, the wine is also given a chance to rid itself of the excess carbon dioxide from fermentation. As the CO2 escapes, oxygen enters the wine with each transfer, helping eventually to age the wine.
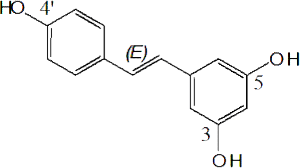 What are the benefits of resveratrol, an antioxidant found in wine?
What are the benefits of resveratrol, an antioxidant found in wine?The Journal of Bilogical Chemistry (J. Biol. Chem., Nov 2005; 280: 37377 - 37382 )reported: "Several epidemiological studies indicate that moderate consumption of wine is associated with a lower incidence of Alzheimer's disease. Wine is enriched in antioxidant compounds with potential neuroprotective activities. However, the exact molecular mechanisms involved in the beneficial effects of wine intake on the neurodegenerative process in Alzheimer's disease brain remain to be clearly defined. Here we show that resveratrol (trans-3,4',5-trihydroxystilbene), a naturally occurring polyphenol mainly found in grapes and red wine, markedly lowers the levels of secreted and intracellular amyloid- (A) peptides produced from different cell lines. Resveratrol does not inhibit A production, because it has no effect on the A-producing enzymes - and -secretases, but promotes instead intracellular degradation of A via a mechanism that involves the proteasome. Indeed, the resveratrol-induced decrease of A could be prevented by several selective proteasome inhibitors and by siRNA-directed silencing of the proteasome subunit 5. These findings demonstrate a proteasome-dependent anti-amyloidogenic activity of resveratrol and suggest that this natural compound has a therapeutic potential in Alzheimer's disease." For a critical look at the potential benefits of resveratol, see this site
Copyright ©2005
|
|
|
|